Method for separating and culturing pig spermatogonial stem cells by mechanical process
A technique for separating and culturing spermatogonial stem cells, applied in the field of mechanical separation and culturing of porcine spermatogonial stem cells, achieving the effects of high success rate, large number of cells, and reduced experimental costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Acquisition and cultivation of porcine spermatogonia
[0031] (1) On the ultra-clean bench, put the piglet’s testes into a petri dish filled with PBS, and wash with PBS for 2 to 3 times to remove blood stains;
[0032] (2) Remove the testicular tunica, remove the connective tissue in the testicular parenchyma with sharp forceps, and cut the remaining testicular tissue into pieces with iris scissors until the tiny tissue pieces are no longer visible, then transfer to a 15 ml centrifuge tube;
[0033] (3) Add 10 times the volume of PBS, pipette gently, then centrifuge at 16°C, 600 rpm for 5 min, and discard the supernatant. Repeat this process for 2-3 times;
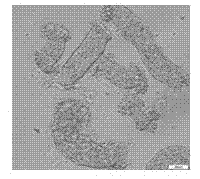
[0034] (4) Adjust the number of seminiferous tubules and inoculate into 25 cm pre-coated with 0.1% gelatin 2 Culture flasks at 37 °C, 5 % CO 2 , saturated humidity sterile incubator conditions ( Figure 1~5 ).
Embodiment 2
[0035] Example 2 Positive detection of alkaline phosphatase (Alkaline Phosphatase, AP)
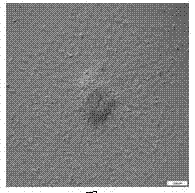
[0036] Wash the PSSCs to be detected once with PBS; fix with 4% paraformaldehyde at room temperature for 20 min, wash with PBS three times, each time for 5 min; add 1 mL of alkaline phosphatase buffer, and add 6.6 μL of NBT and BCIP (vector) 3.3 μl each, make a mixing tube, mix thoroughly; incubate at room temperature with light blocked for 10-15 min, wash with PBS, stop the reaction in time, observe and take pictures under a microscope (such as Figure 6 ).
[0037] Through AP staining, it can be preliminarily judged that the clones obtained by the one-step enzymatic method are PSSCs clones.
Embodiment 3
[0038] Example 3 Immunofluorescence identification and detection of PSSCs
[0039] (1) After the cell slides are prepared, take out the slides and wash them with PBS at 37 ℃ for 3 times, each time for 3-5 s;
[0040] (2) Fix with 4% paraformaldehyde for 10-30 min; wash with PBS twice, 5 min each time;
[0041] (3) 0.5 % Triton perforated and perforated membrane for 15 minutes; rinsed with PBS twice, 5 minutes each time;
[0042] (4) Block with 1% BSA for 30 min (do not wash after blocking);
[0043] (5) Add primary antibody diluted with 1% BSA, overnight at 4°C; wash with PBS twice, 5 min each time;
[0044] (6) Add secondary antibody diluted with 1 % BSA, at 37 ℃ / 1 h; wash with PBS twice, 5 min each time;
[0045] (7) Stain with 10 μg / mL Hochest for 5 min; mount the slides with anti-fade, and take pictures.
[0046] The clonal group obtained by immunofluorescence detection mechanism specifically expresses the important stem cell marker gene Oct4, which proves that it is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com