Method for detecting and analyzing arranon
An analytical method and mobile phase technology, applied in the field of medicine, can solve the problems of patents or public literature reports of non-detection analytical methods, and achieve the effect of high sensitivity and good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
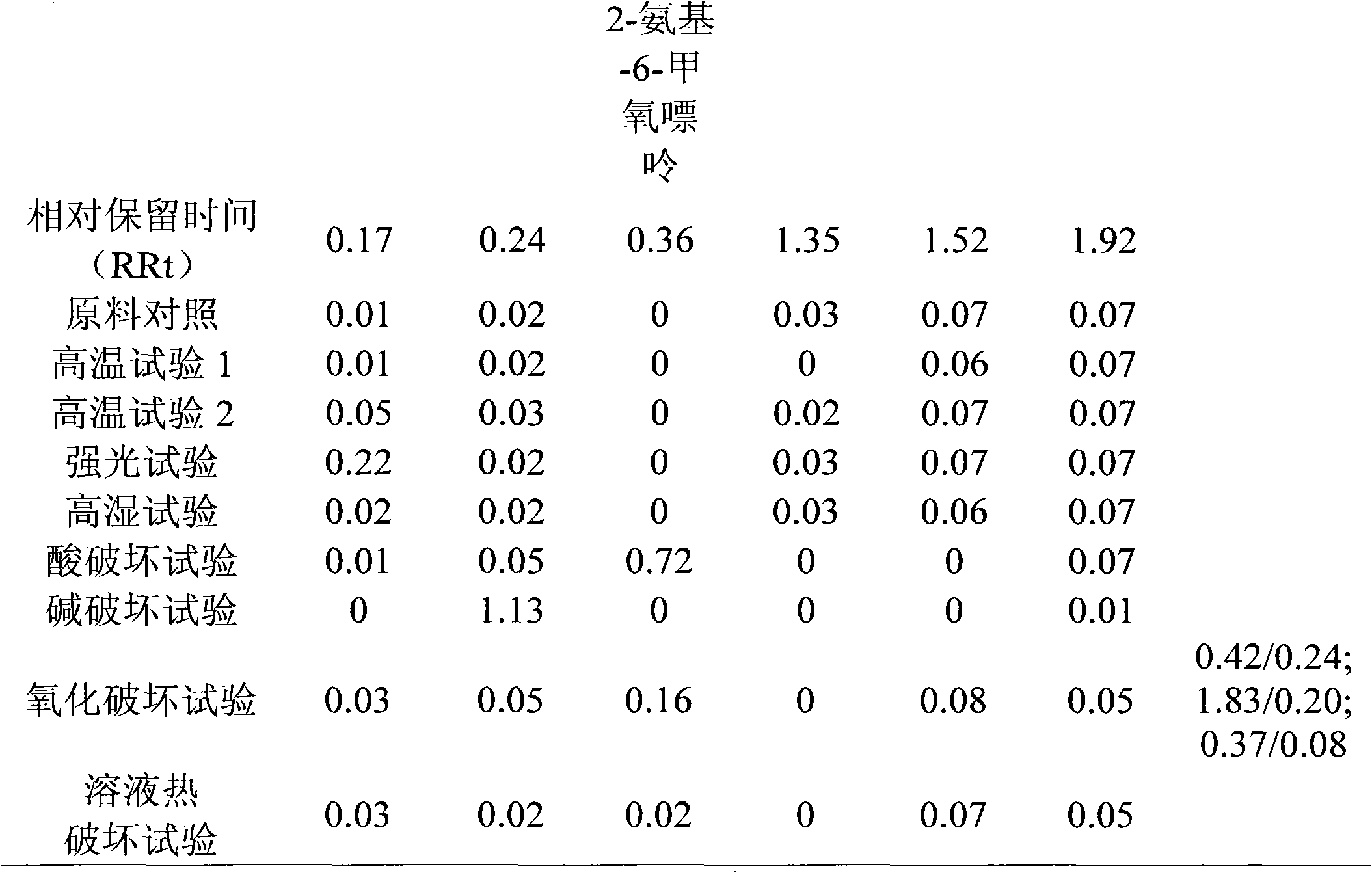
[0075] Measure according to Chinese Pharmacopoeia 2005 edition two appendix VD high performance liquid chromatography, with octadecyl bonded silica gel as filler, ODS pre-column,
[0076] The column temperature is 25°C, the detection wavelength is 210nm, the phosphoric acid solution is used as the mobile phase A, and the acetonitrile-phosphoric acid solution (1:2) is used as the mobile phase B, and the ratio of the mobile phase A is 92%, and the mobile phase B is 0-60 minutes. The proportion is 8%, 60-80 minutes, the proportion of mobile phase A is 70%, the proportion of mobile phase B is 30%, 80-90 minutes, the proportion of mobile phase A is 92%, the proportion of mobile phase B is 8% for gradient elution ;
[0077] Preparation of the test solution: Accurately weigh Nelarabine Injection (batch number: 080806), dilute with water, shake up, as the test solution;
[0078] Precisely draw the test solution and inject it into the liquid chromatograph, and record the chromatogram....
Embodiment 2
[0081] Measure according to Chinese Pharmacopoeia 2005 edition two appendix VD high performance liquid chromatography, with octadecyl bonded silica gel as filler, ODS pre-column,
[0082] The column temperature is 25°C, the detection wavelength is 247nm, the phosphoric acid solution is used as the mobile phase A, and the acetonitrile-phosphoric acid solution (1:1) is used as the mobile phase B, and the ratio of the mobile phase A is 92%, and the mobile phase B is 0-60 minutes. The proportion is 8%, 60-80 minutes, the proportion of mobile phase A is 70%, the proportion of mobile phase B is 30%, 80-90 minutes, the proportion of mobile phase A is 92%, the proportion of mobile phase B is 8% for gradient elution ;
[0083] Preparation of the test solution: Accurately weigh Nelarabine Injection (batch number: 080826), dilute with water, shake up, as the test solution;
[0084] Precisely draw the test solution and inject it into the liquid chromatograph, and record the chromatogram....
Embodiment 3
[0087] Measure according to Chinese Pharmacopoeia 2005 edition two appendix VD high performance liquid chromatography, with octadecyl bonded silica gel as filler, ODS pre-column,
[0088] The column temperature is 25°C, the detection wavelength is 210nm, the phosphoric acid solution is used as the mobile phase A, and the acetonitrile-phosphoric acid solution (2:1) is used as the mobile phase B, and the ratio of the mobile phase A is 92%, and the mobile phase B is 0-60 minutes. The proportion is 8%, 60-80 minutes, the proportion of mobile phase A is 70%, the proportion of mobile phase B is 30%, 80-90 minutes, the proportion of mobile phase A is 92%, the proportion of mobile phase B is 8% for gradient elution ;
[0089] Preparation of the test solution: Accurately weigh an appropriate amount of Nalarabine injection (batch number: 080905), dilute with water, shake well, and use it as the test solution;
[0090] Precisely draw the test solution and inject it into the liquid chrom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com