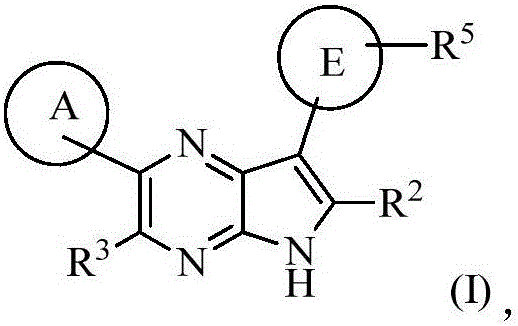
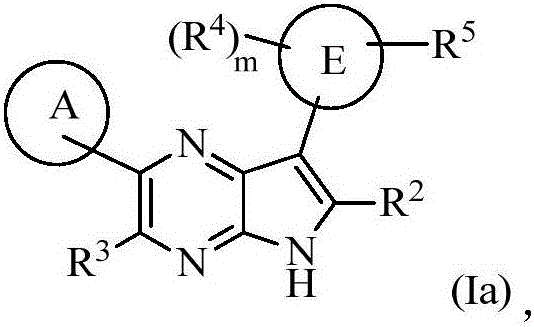
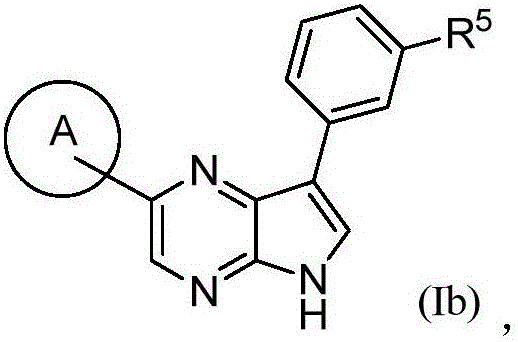
Compounds used as JAK inhibitor, and use of compounds
A technology of compounds and hydrates, applied in the field of medicine, can solve problems such as signal transduction disorders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0574] 3-((3-(2-(1-methyl-1H-pyrazol-4-yl)-5H-pyrrolo[2,3-b]pyrazin-7-yl)phenyl)amino)propionitrile
[0575]
[0576] Step 1: Compound 3-((3-(2-(1-methyl-1H-pyrazol-4-yl)-5-((2-(trimethylsilyl)ethoxy)methyl)- Synthesis of 5H-pyrrolo[2,3-b]pyrazin-7-yl)phenyl)amino)propionitrile
[0577] To 7-bromo-2-(1-methyl-1H-pyrazol-4-yl)-5-((2-(trimethylsilyl)ethoxy)methyl)-5H-pyrrolo[2 ,3-b]pyrazine (30mg, 0.07mmol) in 1,4-dioxane (4mL) solution was added successively 3-((3-(4,4,5,5-tetramethyl-1, 3,2-dioxaborolan-2-yl)phenyl)amino)propionitrile (24mg, 0.08mmol), potassium carbonate (16mg, 0.12mmol), Pd(dppf)Cl 2 (10mg, 0.01mmol) and water (1mL), heated at 110°C for 7 hours under nitrogen atmosphere, diluted with water (10mL), extracted with dichloromethane (15mLx3), dried over anhydrous sodium sulfate, concentrated column chromatography (petroleum ether / ethyl acetate (v / v)=2 / 1) to obtain 26 mg of brown oil, yield: 74.74%.
[0578] MS(ESI,pos.ion)m / z:474.3[M+1] + .
[0579] Ste...
Embodiment 2
[0597] 3-((3-(2-cyclopropyl-5H-pyrrolo[2,3-b]pyrazin-7-yl)phenyl)amino)propanamide
[0598]
[0599] Step 1: Compound 3-((3-(2-cyclopropyl-5-((2-(trimethylsilyl)ethoxy)methyl)-5H-pyrrolo[2,3-b]pyr Synthesis of oxazin-7-yl)phenyl)amino)propionitrile
[0600] Under nitrogen protection, toluene (10 mL) and water (1.2 mL) were added to 7-bromo-2-cyclopropyl-5-((2-(trimethylsilyl)ethoxy)methyl)-5H- Pyrrolo[2,3-b]pyrazine (200mg, 0.42mmol), cyclopropylboronic acid (72mg, 0.85mmol), potassium phosphate heptahydrate (440mg, 1.36mmol), palladium acetate (6mg, 0.02mmol), three In a mixture of cyclohexylphosphine (15mg, 0.04mmol), reflux at 110°C for 19h, the reaction solution was cooled to room temperature, filtered through diatomaceous earth, the filtrate was concentrated under reduced pressure, and subjected to column separation (petroleum ether / ethyl acetate (v / v) = 4 / 1) Obtained 160 mg of light red solid, yield: 87.2%.
[0601] MS(ESI,pos.ion)m / z:434.3[M + 1] + .
[0602] S...
Embodiment 3
[0618] 3-((3-(2-(6-fluoro-1-methyl-1H-indazol-3-yl)-5H-pyrrolo[2,3-b]pyrazin-7-yl)phenyl) Amino)propionitrile
[0619]
[0620] Step 1: Compound 3-((3-(2-(6-fluoro-1-methyl-1H-indazol-3-yl)-5-((2-(trimethylsilyl)ethoxy) Synthesis of methyl)-5H-pyrrolo[2,3-b]pyrazin-7-yl)phenyl)amino)propionitrile
[0621]Under nitrogen protection, 3-((3-(2-bromo-5-((2-(trimethylsilyl)ethoxy)methyl)-5H-pyrrolo[2,3-b]pyrazine -7-yl)phenyl)amino)propionitrile (200mg, 0.42mmol), 6-fluoro-1-methyl-3-(tributylstannane)-1H-indazole (380mg, 0.85mmol), iodide Copper (16mg, 0.08mmol) and tetrakistriphenylphosphine palladium (26mg, 0.02mmol) were added together, then DMF (15mL) was added, the temperature was raised to 90°C for 4.5h, the reaction liquid was cooled to room temperature, ethyl acetate (50mL ×3) extraction, with anhydrous Na 2 SO 4 Drying, removal of solvent, and column separation (petroleum ether / ethyl acetate (v / v)=4 / 1) gave 200 mg of light yellow oil, yield: 84.7%.
[0622] MS(ESI...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com