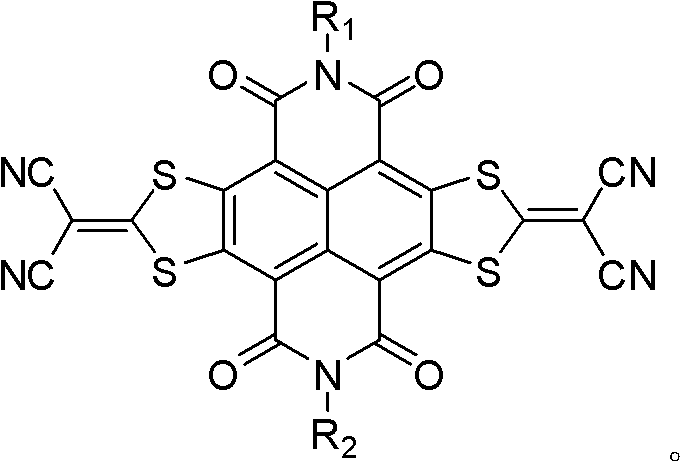
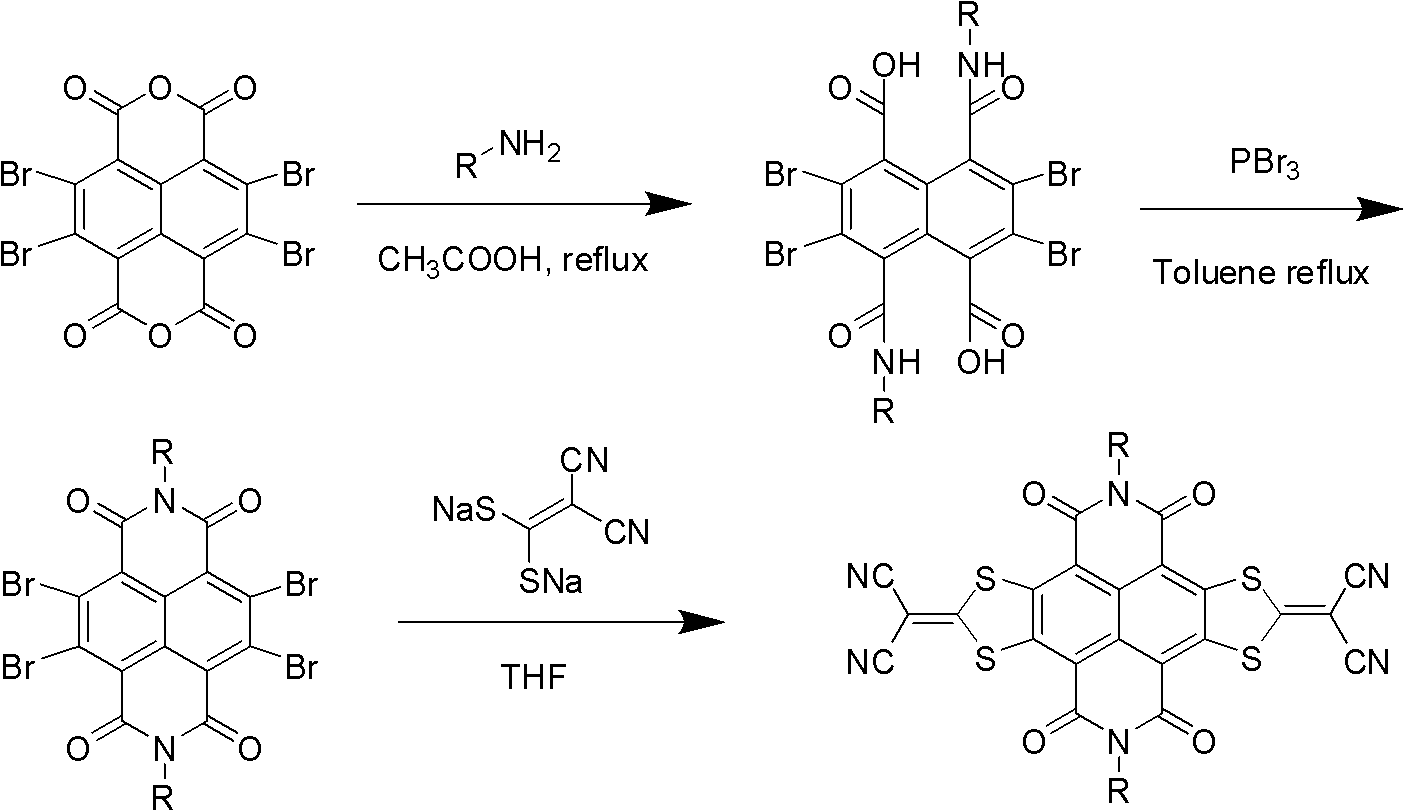
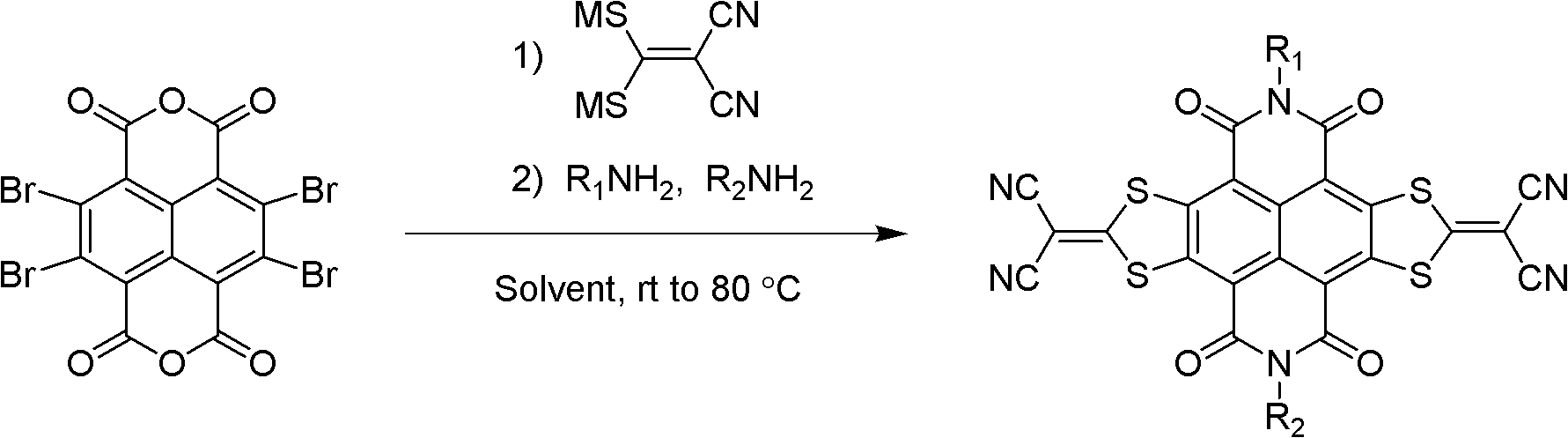
Method for preparing sulfur heterocyclic condensed naphthalimide derivants in one-pot method
A technology of naphthalene diimide and sulfur heterocycle, which is applied in the field of one-pot preparation of sulfur heterocycle fused naphthalene diimide derivatives, which can solve the problem of low yield or only a small amount of product or no product products, the inability to achieve effective synthesis of compounds, long synthesis steps, etc., to achieve the effects of rich variety, easy synthesis and preparation, and low synthesis cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-9
[0056] (1) Example 1-9: Three-component one-pot preparation method of compound 1-9 (symmetrical N-substituted NDI-DTYM2 derivatives)
Embodiment 1
[0057] Example 1: N, N'-bis(2-octyl-dodecyl)-[2,3-d:6,7-d']-bis[2-(1,3-dithiacyclopenta Synthesis of alken-2-ylidene)-2-propanedicyano]-naphthalene-1,4,5,8-tetracarboxylic diimide (1).
[0058]
[0059] Concrete synthetic steps are:
[0060] Under nitrogen protection, 2,3,6,7-tetrabromonaphthalene tetracarboxylic dianhydride (TBNDA) (100mg, 0.17mmol) and 1,1-dicyanoethylene-2,2-dithiolate sodium (96mg , 0.52mmol) was added into 15mL DMF and heated to 50°C. After stirring for 1 h, 2-octyldodecylamine (204 mg, 0.68 mmol) was added, and the temperature was maintained for 7 h. Cool to room temperature, pour the reaction solution into 100 mL of saturated ammonium chloride solution, filter, wash the filter residue with water, dry in vacuo, use dichloromethane / petroleum ether (2:1) as eluent, and use a silica gel chromatography column to analyze the crude The product was separated and purified to obtain 52 mg of brown-red solid 3 with a yield of 28%. Mass Spectrum: MS (MALDI-T...
Embodiment 2
[0061] Example 2: N, N'-bis(2-decyl-tetradecyl)-[2,3-d:6,7-d']-bis[2-(1,3-dithiacyclopenta Synthesis of alken-2-ylidene)-2-propanedicyano]-naphthalene-1,4,5,8-tetracarboxylic diimide (2).
[0062]
[0063] Using 2-decyltetradecylamine instead of 2-octyldodecylamine, the synthesis method is the same as in Example 1 to obtain compound 2 as a brownish-red solid with a yield of 26%. Mass Spectrum: MS (MALDI-TOF) m / z 1214.9 (M + ); H NMR spectrum: 1 H-NMR (300MHz, CDCl 3 )δ (ppm): 0.848-0.889 (m, 6H, -CH 3 ), 1.232-1.311 (br, 40H, -CH 2 -), 2.013(m, 1H, CH), 4.222-4.247(d, 2H, J=7.2Hz, -CH 2 -N); Carbon NMR spectrum: 13 C-NMR (100Hz, CDCl 3 ):δ14.127,22.690,26.303,29.367,29.595,29.670,29.699,30.040,31.505,31.924,36.604,46.538,71.007,111.551,117.806,125.112,145.258,161.965(C=O),182.038(=CS 2 ).Elemental analysis calculation value (Anal.Calcd.For)C 70 h 98 N 6 o 4 S 4 : C, 69.15; H, 8.12; N, 6.91; Found: C, 69.36; H, 7.89; N, 6.78.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com