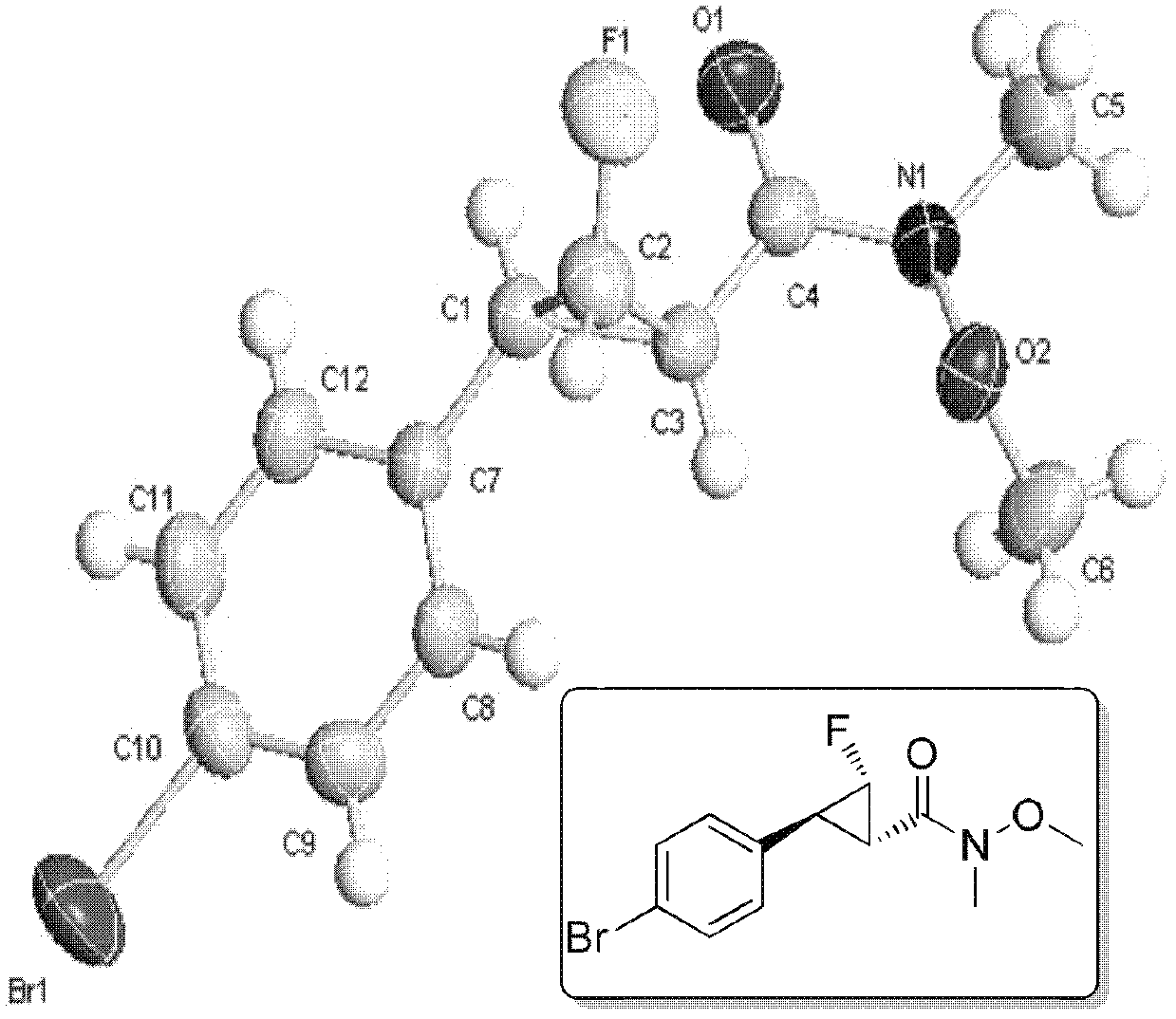
Monofluorocyclopropane compounds as well as preparation method and application for same
A compound and alkyl technology, which is applied in the field of monofluorocyclopropane compounds and their preparation, can solve the problems of poor substrate universality, low conversion rate, poor enantioselectivity and diastereoselectivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
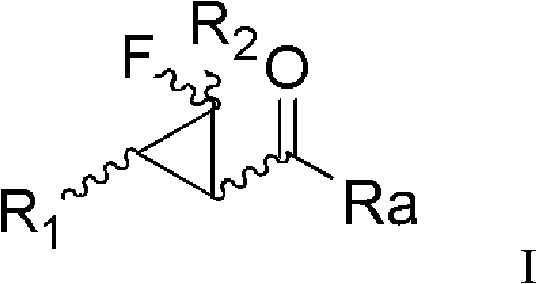
[0134] Method for preparing compound of formula I
[0135] The method for preparing the compound of formula I provided by the present invention includes the steps:
[0136] (a1) The compound of formula IV is reacted with a fluorinating reagent to obtain a compound of formula II;
[0137] Or (a2) the compound of formula VI is reacted with an alkylating reagent to obtain a compound of formula II;
[0138]
[0139] (b) In an organic solvent and in the presence of a base, the compound of formula III reacts with the compound of formula II to form the compound of formula I,
[0140]
[0141] In each formula, R 1 , R 2 , Ra, Rb, R 4 The definition of is as before;
[0142] In another preferred embodiment, the fluorinating reagent may be a fluorinating reagent commonly used in the art. For example, the fluorinating reagent includes but not limited to: NFSI fluorinating agent, Selectfluor fluorine reagent, NFTh fluorinating agent, and NFOBS fluorine reagent. Chemical agent, nitrogen-fluoropyrid...
Embodiment 1
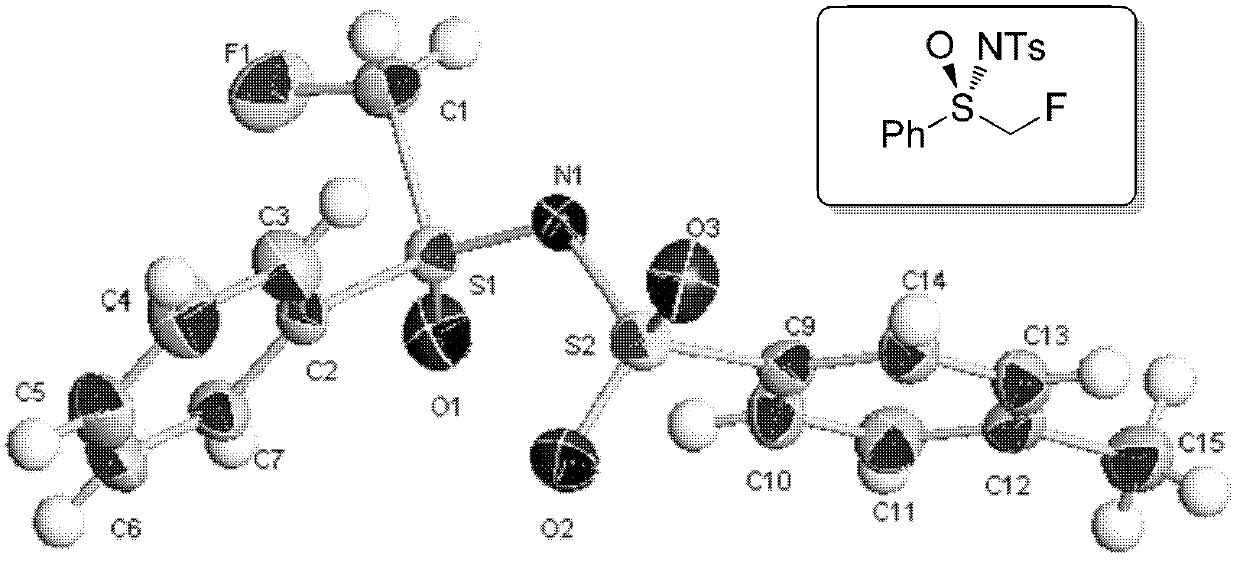
[0184] Synthesis of [(R)-N-p-toluenesulfonyl]monofluoromethyl phenyl sulfoximine ((R)-N-Tosyl-S-monofluoromethyl-S-phenylsulfoximine)
[0185]
[0186] Under the protection of nitrogen, 9.3 g (30 mmol) of [(S)-N-p-toluenesulfonyl]methylphenylsulfoximine and 150 ml of dry THF were added to a 250 ml three-necked flask, cooled to -78°C, and then NBuLi (1.6M, 33mmol, 1.1 equiv) was added dropwise, and after reacting at -78°C for half an hour, NFSI (12g, 1.2 equiv) was added. Spontaneously heat up to 25℃ and stir overnight, then add H 2 The reaction was quenched by O, and the organic layer was obtained by extraction with ether. The organic layer was washed with saturated brine, and MgSO was added 4 After drying, the solvent was evaporated and the product was obtained by column chromatography (6.4 g, yield 65%).
[0187] White solid. Melting point: 89-91°C. Optical rotation: [α] D 24 =+49.8°(c=1.00, CHCl 3 ). The enantiomeric excess was determined by a chiral column Lux 5u Amylose-2 (...
Embodiment 2
[0190] Synthesis of [(R)-N-p-toluenesulfonyl]-1-fluoroethylphenylsulfoximine ((R)-N-Tosyl-S-1-fluoroethy-S-phenylsulfoximine)
[0191]
[0192] Under the protection of nitrogen, add 1.96 g (6 mmol) of [(R)-N-p-toluenesulfonyl]fluoromethylphenylsulfoximine and 30 ml of dry THF into a 50 ml three-necked flask, and cool to -78°C. Then nBuLi (1.6M, 6.6mmol, 1.1equiv) was added dropwise, and after reacting at -78°C for half an hour, MeI (2.13g, 2.5equiv) was added. After reacting at -78℃ for 1 hour, the temperature was raised to 25℃ and stirred overnight, then H was added 2 The reaction was quenched by O, and the organic layer was obtained by extraction with ether. The organic layer was washed with saturated brine, and MgSO was added 4 After drying, the solvent was evaporated, and the product was obtained by column chromatography (1.54 g, yield 75%). One of the diastereoisomer data:
[0193] White solid. Melting point: 117-118°C. Optical rotation: [α] D 28 = +141.7° (c = 0.52, CHCl 3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com