Method for diagnosis of cancer and monitoring of cancer treatments
A cancer and liver cancer technology, applied in the field of cancer diagnosis and cancer treatment monitoring, can solve the problems of lack of specificity and hindering the clinical application of cancer diagnosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Embodiment 1: Method of sample analysis and DNA fragment pattern selection
[0071] In general, sample analysis and fragment mode selection are performed in the following five steps. Step (1) isolation of circular DNA, step (2) quantification of isolated DNA, step (3) quantification of DNA fragments by real-time PCR, step (4) data evaluation and fragmentation pattern selection and finally step (5) DNA fragmentation index ( DFI) calculation.
[0072] Step (1): Isolation of circular DNA
[0073] According to the manufacturer's instructions (Qiagen kit manual, third edition, March 2007, pages 23-25), the circular DNA was extracted from 1 to 2.5ml of frozen (-20°C) or fresh serum samples. Frozen plasma samples (2- 2.5ml).
[0074] Step (2): Quantification of isolated DNA
[0075] The isolated DNA (present in a total volume of up to 20 μl) was quantified using the PicoGreen assay (Cat. No. P11496, Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructi...
Embodiment 2
[0120] Example 2: Recurrence Diagnosis of Metastatic Breast Cancer by DNA Fragmentation Analysis
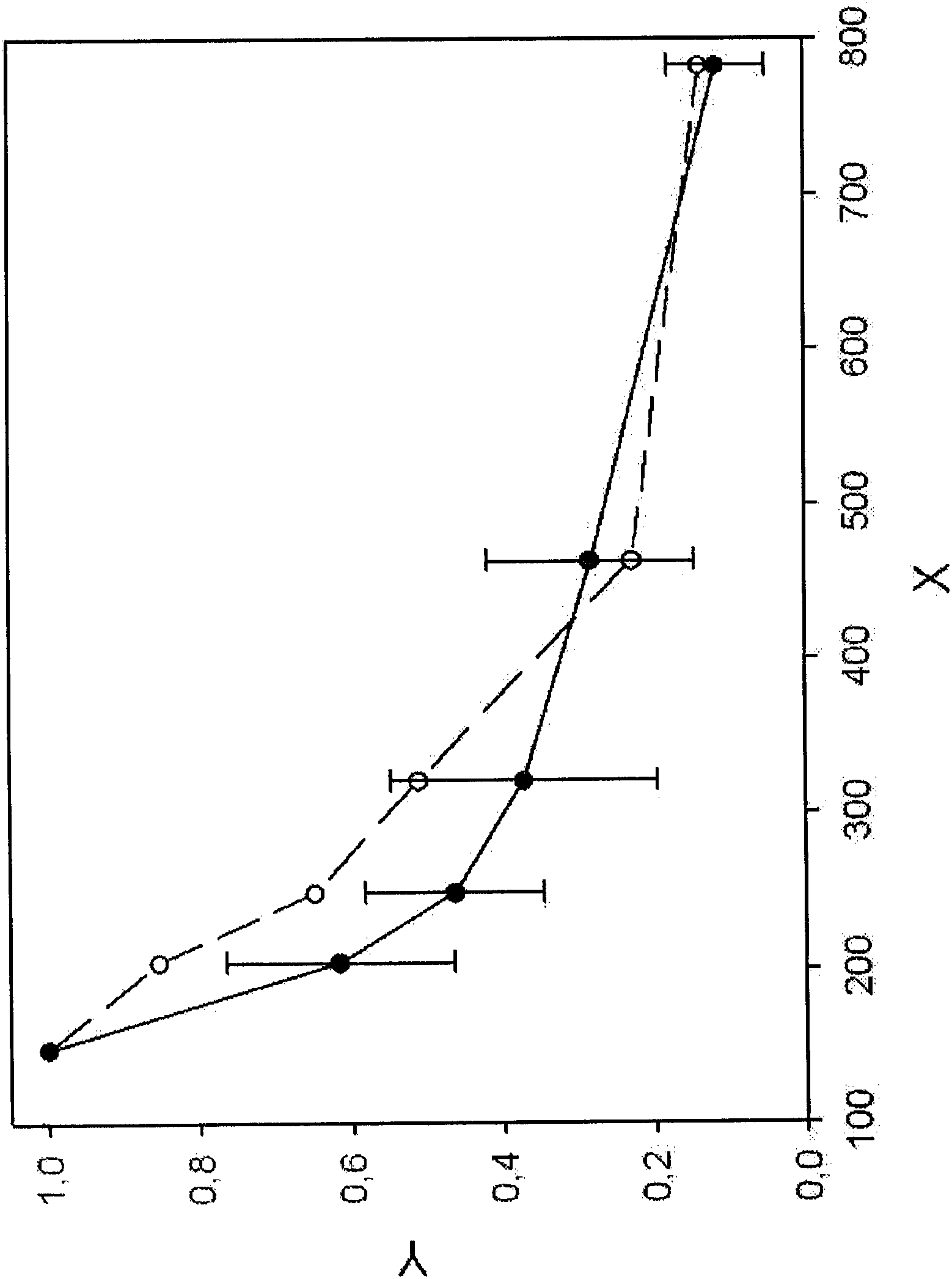
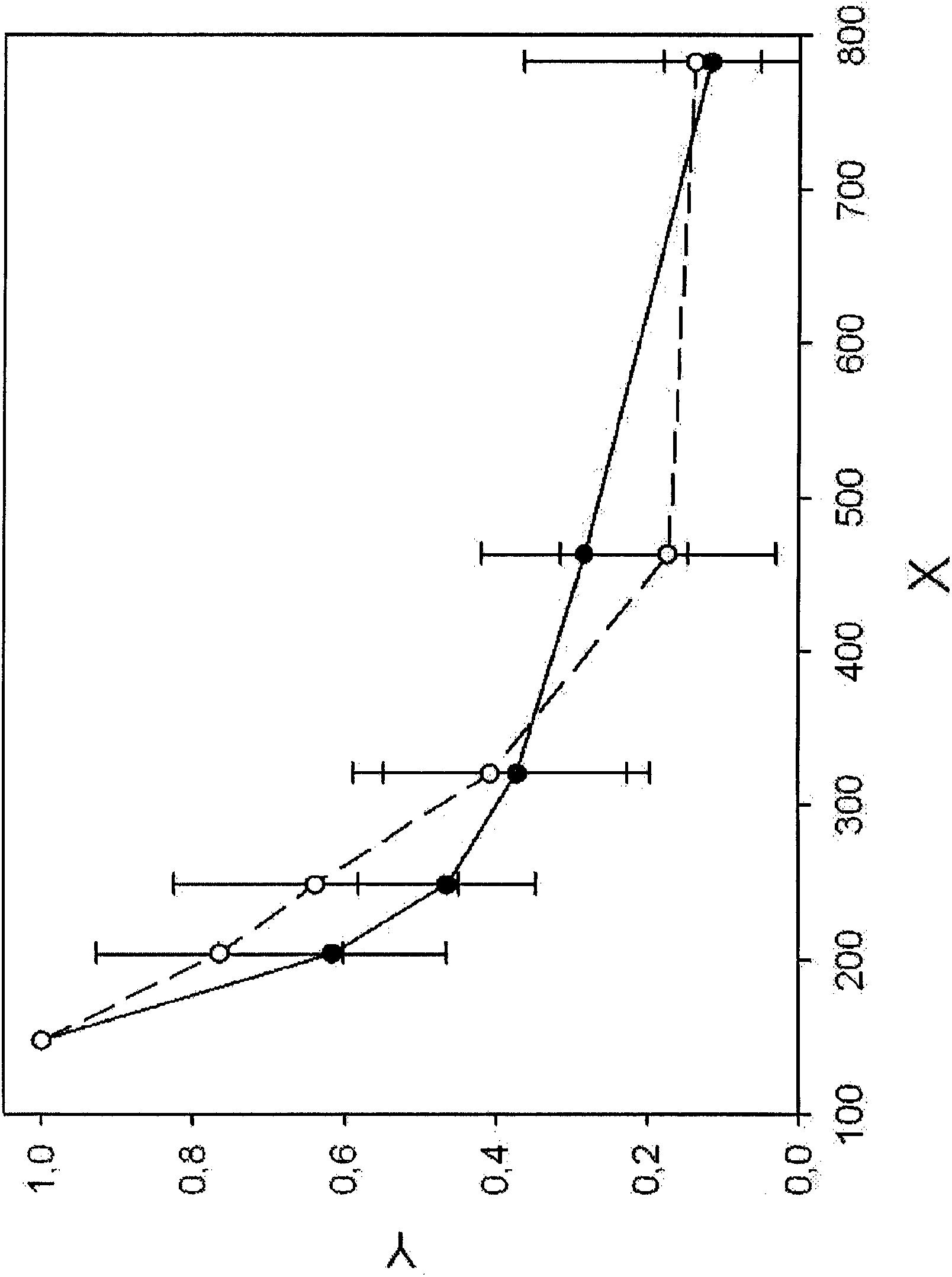
[0121] DNA fragmentation patterns were recorded for healthy donors (n=17) and a collection of metastatic breast cancer patients (n=10) at different clinical stages (Example 1). LINE1 fragments of indicated bp length (see Table 2) were analyzed and normalized and plotted (see Figure 1B ). DNA fragmentation patterns of pooled breast cancer sera (see Figure 1A ) indicates elevated levels of DNA fragments ranging from 200 to 400 bp compared to healthy controls.
[0122] According to formula [2] (using LINE1-B, LINE1-C, LINE1-E and LINE1-F, see Table 2) and formula [4] (using LINE1-B, LINE1-C, LINE1-D, LINE1-E and LINE1-F, see Table 2), the DNA Fragmentation Index (DFI) was calculated for each patient / collection.
[0123] Mean DFI for healthy controls compared to 0.64 for the breast cancer set 4 (Equation [2]) was 0.02 ± 0.01,; thus suggesting a potential cutoff of approximately...
Embodiment 3
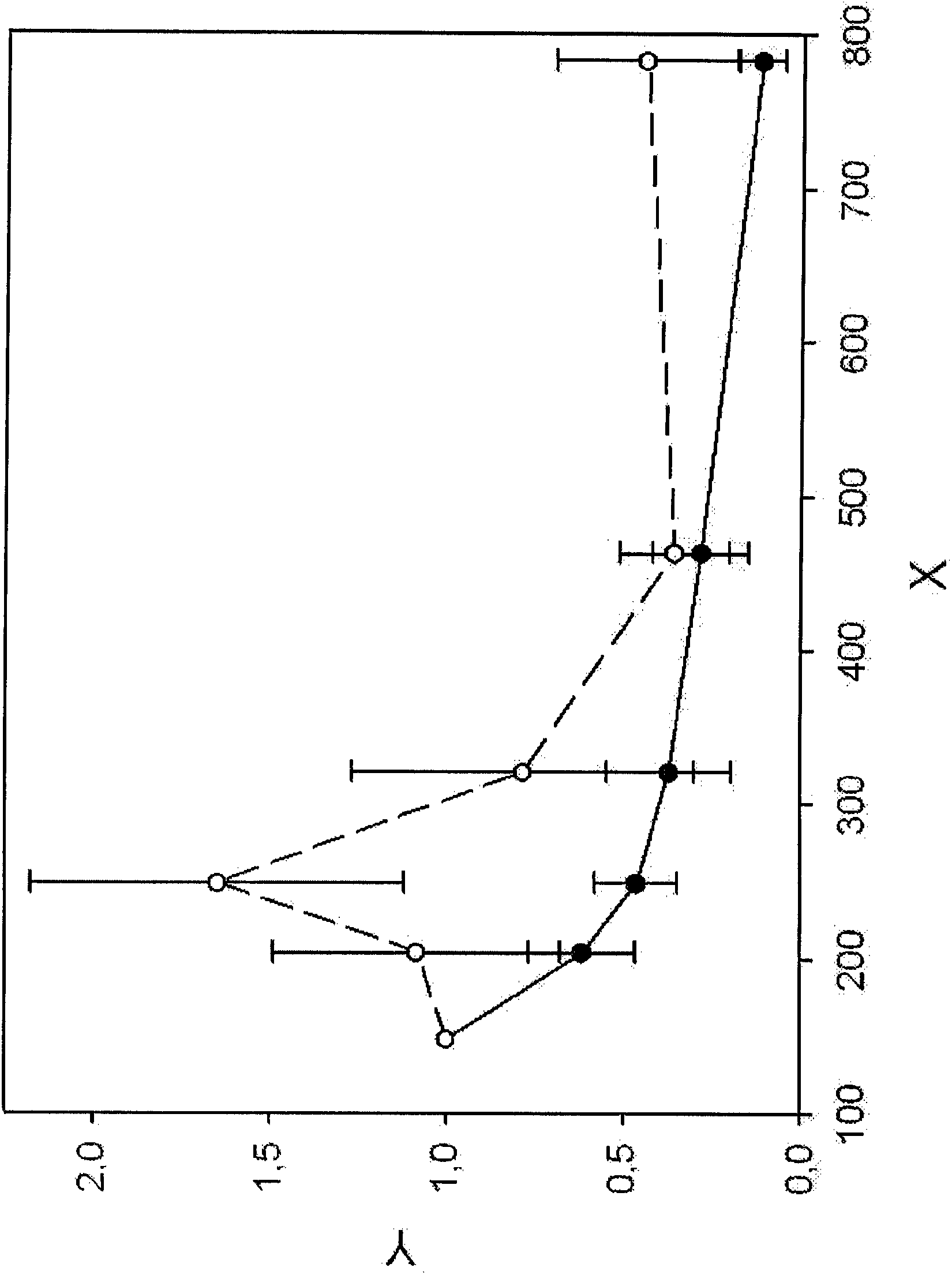
[0134] Example 3: Diagnosis of Hepatocellular Carcinoma by DNA Fragmentation Analysis
[0135] DNA fragmentation patterns were recorded as described in Example 1 for cirrhotic patients (at-risk patients) and HCC patients. LINE1 fragments of the indicated sizes (see Table 2) were analyzed and normalized and plotted (see figure 2 ). figure 2 showed significant differences in DNA fragmentation pattern profiles in HCC patients compared with at-risk patients with cirrhosis. Therefore, a diagnosis of HCC can be obtained by recording the DNA fragmentation pattern in the indicated range of 148 to 783 bp but at least in the range of 148 to 463 bp and comparing it with DNA fragmentation in patients with cirrhosis and / or chronic hepatitis C Schema comparison. The DNA fragmentation pattern differed most significantly between HCC patients and controls between 204 and 463 bp.
[0136] DFI is calculated according to formulas [1], [2] and [4.1]. To optimize the LINE1 model, the differe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com