Efficient sulfur solving agent for sulfur deposits in sulfur-containing gas well
A technology of depositing sulfur and sulfur dissolving agent, which is applied in the direction of drilling compositions, chemical instruments and methods, etc., can solve the problems of high cost, small amount of dissolved sulfur, and slow speed of dissolving sulfur, and achieve low cost and high dissolved sulfur Large, fast sulfur dissolving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
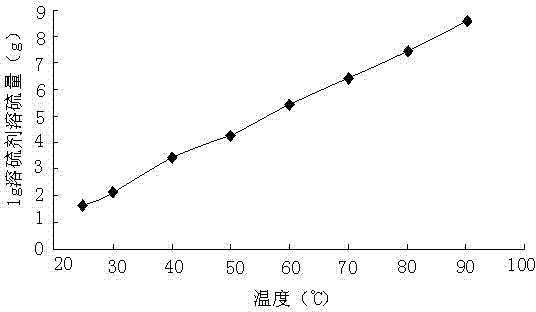
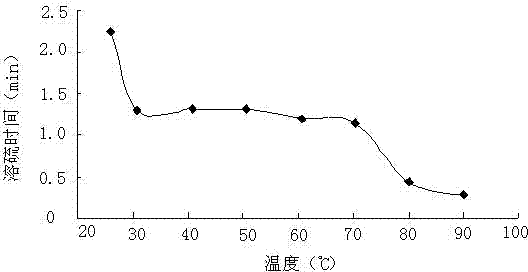
[0045] First configure the aqueous sodium hydroxide solution, dissolve 3g of sodium hydroxide in 10g of water, after the sodium hydroxide is completely dissolved, add 0.08g of sodium hydrosulfide, and after it is completely dissolved, add 10g of dimethyl disulfide, 0.5 g N, N-dimethylformamide to obtain a sulfur-dissolving agent. At 25, 30, 40, 50, 60, 70, 80, and 90°C, the experiment of dissolving sublimated sulfur powder was carried out, and the results are shown in figure 1 . At 25, 30, 40, 50, 60, 70, 80, and 90°C, half of the maximum amount of sulfur dissolved at each temperature was dissolved, and the sulfur dissolution time experiment was carried out. see results figure 2 .
[0046] Experimental method and steps:
[0047] The experiment was carried out at a pressure of 1 atm and a certain temperature. Set the mass to M 0 The sublimated sulfur powder is added to the sulfur-dissolving agent with a mass of M, and the stirrer rotates at a speed of 280r / min. After ful...
Embodiment 2
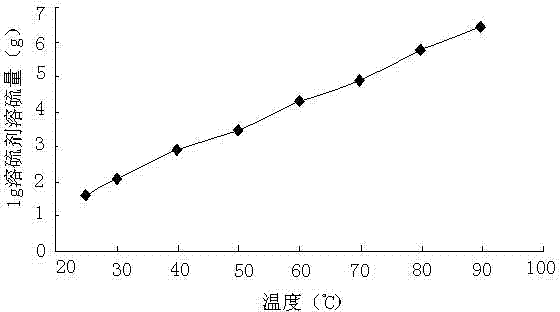
[0049] First configure the sodium hydroxide aqueous solution, dissolve 10g of sodium hydroxide in 25g of water, after the sodium hydroxide is completely dissolved, add 0.2g of sodium hydrosulfide, and then add 10g of dimethyl disulfide, 1.5 g N, N-dimethylformamide to obtain a sulfur-dissolving agent. At 25, 30, 40, 50, 60, 70, 80, and 90°C, the experiment of dissolving sublimated sulfur powder was carried out, and the results are shown in image 3 . At 25, 30, 40, 50, 60, 70, 80, and 90°C, half of the maximum amount of sulfur dissolved at each temperature was dissolved, and the sulfur dissolution time experiment was carried out. see results Figure 4 .
[0050] The experimental method and steps are the same as in Example 1.
Embodiment 3
[0052] First configure the aqueous sodium hydroxide solution, dissolve 2.5g of sodium hydroxide in 7g of water, after the sodium hydroxide is completely dissolved, add 0.06g of sodium hydrogen sulfide, and then add 10g of dimethyl disulfide, 0.6 g of N,N-dimethylformamide to obtain a sulfur-dissolving agent. At 25, 30, 40, 50, 60, 70, 80, and 90°C, the experiment of dissolving sublimated sulfur powder was carried out, and the results are shown in Figure 5 . At 25, 30, 40, 50, 60, 70, 80, and 90°C, half of the maximum amount of sulfur dissolved at each temperature was dissolved, and the sulfur dissolution time experiment was carried out. see results Image 6 .
[0053] The experimental method and steps are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com