Application of monoclonal antibody for treating colorectal cancer
A monoclonal antibody, colorectal cancer technology, applied in the direction of antibodies, anti-tumor drugs, drug combinations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
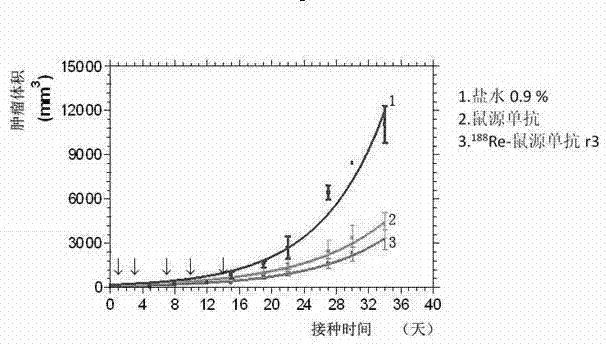
[0035] Embodiment 1, preclinical pharmacodynamics study
[0036] 1. In vitro activity
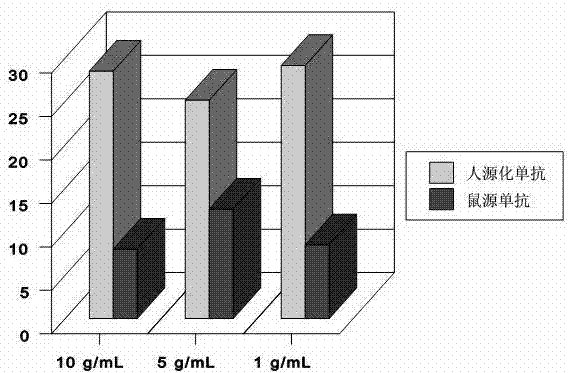
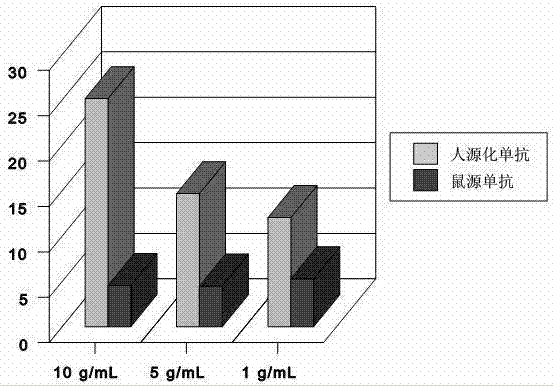
[0037] Nimotuzumab was determined by an equilibrium binding assay between 99mTc-labeled Nimotuzumab and the H-125 human lung adenocarcinoma tumor cell line, the A431 human vaginal epithelial carcinoma tumor cell line, and the MDA-MB-468 human breast cancer tumor cell line Ability of mAbs to bind tumor cells. After labeling with a freeze-drying kit (purchased from Sigma-Aldrich (Shanghai) Trading Co., Ltd.) at room temperature for 15 minutes, an average of (97.38±0.23)% of 99mTc was labeled on the IgG antibody. Fast paper chromatography of mAb in acetone showed that approximately 3% or less of free pertechnetic acid ran to the reagent front (Rf = 10). This revealed that almost all of the pertechnetic acid was reduced. The colloidal form of Nimotuzumab was (1.68±0.81)% as determined by the ITLC method impregnated with 1.0% albumin. The maximum binding (Bmax) of 99mTc-labeled nimotuzumab c...
Embodiment 2
[0057] Embodiment 2, safety test
[0058] The safety trial study of colorectal cancer was divided into 3 dose groups (200, 400, 600 mg), 8 patients in each group, 24 cases in total. To evaluate the tolerability of nimotuzumab combined with chemotherapy in advanced colorectal cancer. Treatment with nimotuzumab combined with irinotecan. The completion of the treatment cycle evaluates the disease control rate, objective response rate, overall survival, quality of life QoL and safety of nimotuzumab combined with chemotherapy in the treatment of advanced colorectal cancer to investigate nimotuzumab as a potential treatment drug for advanced colorectal cancer security. A total of 24 patients, 13 males and 11 females. The mean age was 59.33 years old (35 to 74 years old), all of them were advanced epithelial cancers. 24 patients were divided into 3 treatment groups, 8 people in each group. The doses of each group are as follows: Group 1=200mg: 8 patients; Group 2=400mg: 8 patien...
Embodiment 3
[0059] Embodiment 3, toxicity test
[0060] 1. Acute Toxicology Study
[0061] The toxicity test of single dose injection was carried out on Sprague Dawley rats in accordance with the requirements of GLP standard. The doses are 0, 0.25, 1.25 and 5 times of the highest human dose of 400 mg (5.71 / mg / Kg), respectively. Each of the four groups of animals consisted of 5 female and 5 male rats. There were no deaths and no clinical abnormalities in the treated and control groups except for an increase in the number of soft stools in all animals (p<0.05). Conclusion: No obvious toxic reaction characteristics were found in injection of any dose of the drug.
[0062] 2. Subacute Toxicity Study
[0063] Repeated-dose subacute studies of nimotuzumab were performed on animals (two rat experiments and one monkey experiment) using doses that reached 4.0 to 12.7 times the highest human dose per kilogram (315 mg / dose or 4.5 mg / Kg). ), the mAb was produced from the main cell bank and the e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com