Spray pyrolysis battery manufacturing method of double-layer film all-solid-state film lithium battery
A double-layer film and double-spray gun technology is applied in the direction of electrolyte battery manufacturing, final product manufacturing, and sustainable manufacturing/processing. Effects of reducing stress, increasing conductance, and reducing influence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
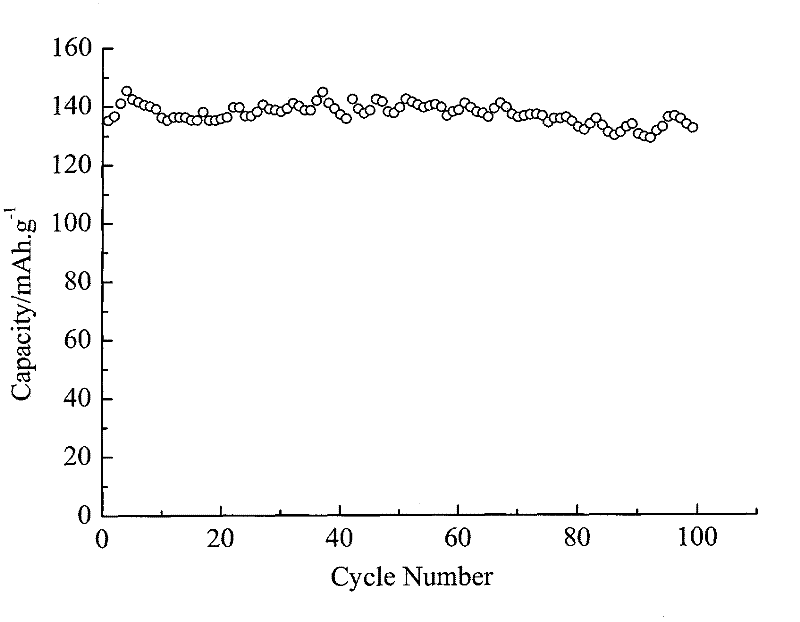
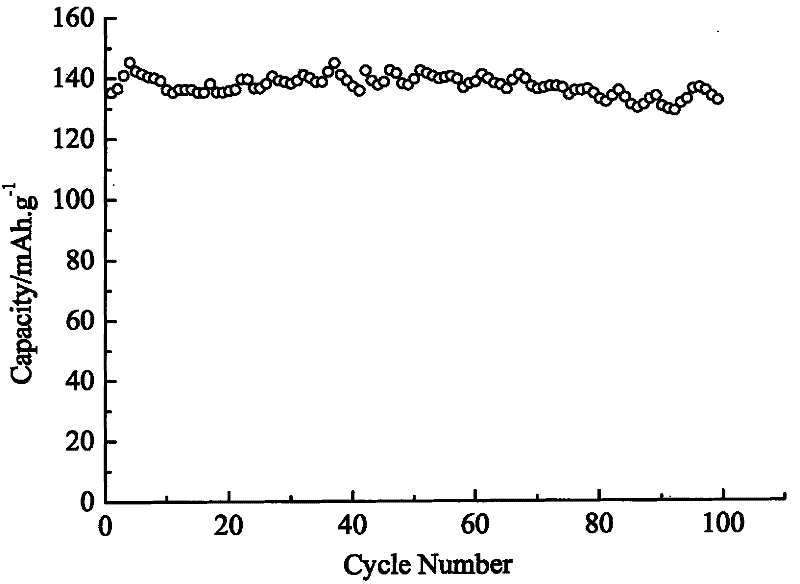
Image
Examples
Embodiment 1
[0019] Example 1: Place the copper substrate on the surface of a stainless steel heating plate at a constant temperature of 450°C, and connect the spray gun A to the precursor solution I: ammonium metavanadate NH 4 VO 3 (0.3mol / L), ammonium chloride NH 4 Cl(0.08mol / L), lithium acetate Li(CH 3 COO) (0.3mol / L), lanthanum nitrate La(NO 3 ) 3 (0.2mol / L), n-butyl titanate Ti(OC 4 h 9 ) 4 (0.4mol / L), CH acetate 3 COOH (0.2mol / L), and ethylene glycol methyl ether (0.1wt%) aqueous solution. Gun B is connected to Precursor Solution II: Lithium Acetate Li(CH 3 COO) (0.4mol / L), n-butyl titanate Ti(OC 4 h 9 ) 4 (0.5mol / L), CH acetate 3Aqueous solution of COOH (0.3mol / L) and ethylene glycol methyl ether (0.15wt%). Spray gun A is 10 cm vertically away from the working surface, and the angle of intersection with the working surface is 65°. The carrier gas atomizes and sprays precursor solution I with a flow rate of 5 mL / min at a pressure of 100 KPa for 30 minutes to the substrat...
Embodiment 2
[0020] Example 2: Place the copper substrate on the surface of a stainless steel heating plate at a constant temperature of 300°C, and connect the spray gun A to the precursor solution I: ammonium metavanadate NH 4 VO 3 (0.4mol / L), ammonium chloride NH 4 Cl(0.09mol / L), lithium acetate Li(CH 3 COO) (0.51mol / L), lanthanum nitrate La(NO 3 ) 3 (0.4mol / L), n-butyl titanate Ti(OC 4 h 9 ) 4 (0.8mol / L), CH acetate 3 COOH (0.2mol / L), and n-pentanol (0.5wt%) aqueous solution. Gun B is connected to Precursor Solution II: Lithium Acetate Li(CH 3 COO) (0.3mol / L), n-butyl titanate Ti(OC 4 h 9 ) 4 (0.375mol / L), acetic acid CH 3 Aqueous solution of COOH (0.2mol / L) and ethylene glycol methyl ether (0.2wt%). Spray gun A is 12 cm vertically away from the working surface, and the angle of intersection with the working surface is 70°. The carrier gas atomizes and sprays the precursor solution I with a flow rate of 6 mL / min at a pressure of 80 KPa for 40 minutes to the substrate. Then...
Embodiment 3
[0021] Example 3: Place the nickel sheet substrate on the surface of a stainless steel heating plate at a constant temperature of 500°C, and connect the spray gun A to the precursor solution I: ammonium metavanadate NH 4 VO 3 (0.45mol / L), ammonium chloride NH 4 Cl(0.1mol / L), lithium acetate Li(CH 3 COO) (0.95mol / L), lanthanum nitrate La(NO 3 ) 3 (0.8mol / L), n-butyl titanate Ti(OC 4 h 9 ) 4 (1.6mol / L), CH acetate 3 COOH (0.3mol / L), and polyvinyl alcohol (0.7wt%) aqueous solution. Gun B is connected to Precursor Solution II: Lithium Acetate Li(CH 3 COO)(0.2mol / L), n-butyl titanate Ti(OC 4 h 9 ) 4 (0.25mol / L), CH acetate 3 Aqueous solution of COOH (0.3mol / L) and ethylene glycol methyl ether (0.2wt%). Spray gun A is 15 cm away from the working surface vertically, and the angle of intersection with the working surface is 80°. The carrier gas atomizes and sprays the precursor solution I with a flow rate of 10 mL / min at a pressure of 150 KPa for 80 minutes to the substra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com