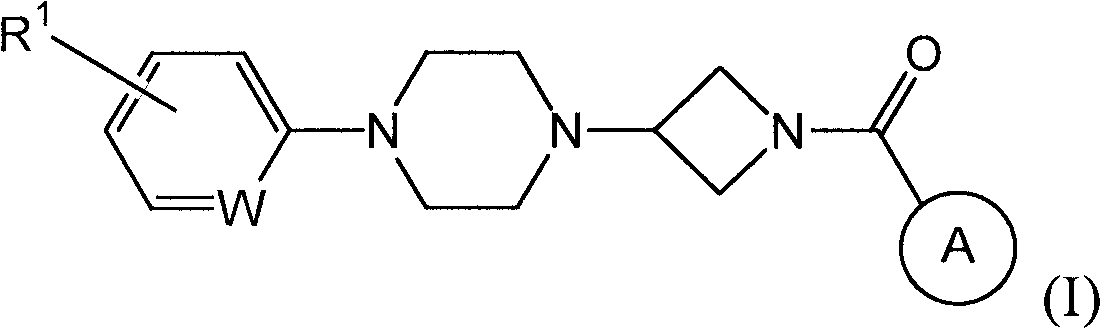
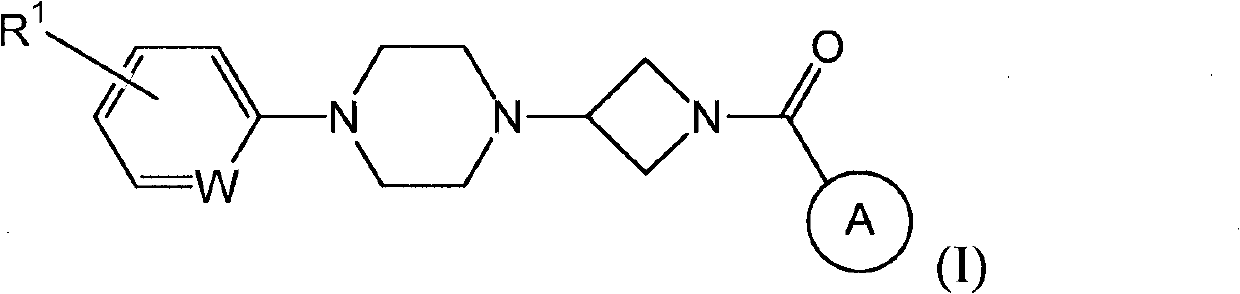
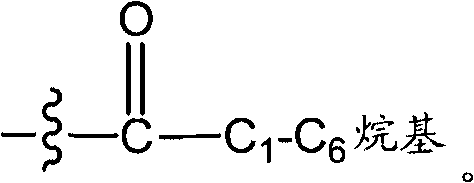Heteroaromatic and aromatic piperazinyl azetidinyl amides as monoacylglycerol lipase inhibitors
A technology of isobutyl and phenylmethyl is applied in preparations for in vivo tests, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve the problem of separating beneficial effects from harmful side effects Kailai and other questions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 2
[0226] Example 2: (in vitro detection and analysis): MGL enzyme activity assay
[0227] All rat-based assays were performed in black 384-well polypropylene PCR microplates (Abgene) in a total volume of 30 μL. The substrate 4-methylumbelliferyl butyrate (4MU-B, Sigma) and the purified mutant MGL enzyme (mut-MGLL 11-313 L179S L186S) were diluted to 150 mM NaCl and 0.001% Tween 20, respectively. 20mM PIPES buffer (pH 7). The compound of formula (I) was pre-dispersed (50 nL) into the assay plate with a Cartisian Hummingbird pipette (Genomic Solutions (Ann Arbor, MI)), and then 4MU-B (25 μL of a 1.2X solution at a final concentration of 10 μM) was added, Enzyme (5 μL of a 6X solution at a final concentration of 5 nM) was then added to initiate the reaction. Final compound concentrations ranged from 17 to 0.0003 [mu]M. Excitation and emission wavelengths of 335nm and 440nm at 37°C, respectively, and a bandwidth of 10nm (Safire 2 , Tecan) to monitor the change in fluorescence d...
example 4
[0233] Example 4: 2-AG Accumulation Assay
[0234] HeLa cells were homogenized with a Polytron in 10 ml (about 400 million cells) of HEPES buffer (HEPES 20 mM, pH 7.4, NaCl 125 mM, EDTA 1 mM, KCl 5 mM, glucose 20 mM). Homogenates from 0.2 billion cells (0.5 ml) were incubated with MGL inhibitors for 15 minutes to block MGL activity, followed by incubation of HEPES buffer with calcium (10 mM) for 20 minutes. The total reaction volume was 5ml. The reaction was terminated by 6 mL of organic solvent extraction (2:1 chloroform / methanol). Methylarachidonyl fluorophosphonate (MAFP) was used as a positive control. In the absence of MAFP, 2-AG levels were about 3.4 pmol / sample. In the presence of 100 nM MAFP, 2-AG levels increased to 174 pmol / sample. The accumulated 2-AG in the organic phase was measured by HPLC / MS according to the following formula: %MAFP=(compound 2-AG / MAFP 2-AG)×100.
[0235] Representative compounds of formula (I) were tested according to the procedures desc...
example 5
[0238] Example 5: Oral Formulations - Hypothetical Example
[0239] As a specific example of the oral composition, 100 mg of Compound #1 prepared in Example 1 was formulated with well-pulverized lactose to provide a total amount of 580-590 mg to fill O-size hard capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com