Method for preparing citraconic anhydride and method for isomerizing/dehydrating itaconic acid
A technology of citraconic anhydride and itaconic acid, applied in chemical instruments and methods, chemical/physical processes, metal/metal oxide/metal hydroxide catalysts, etc. Recycling and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
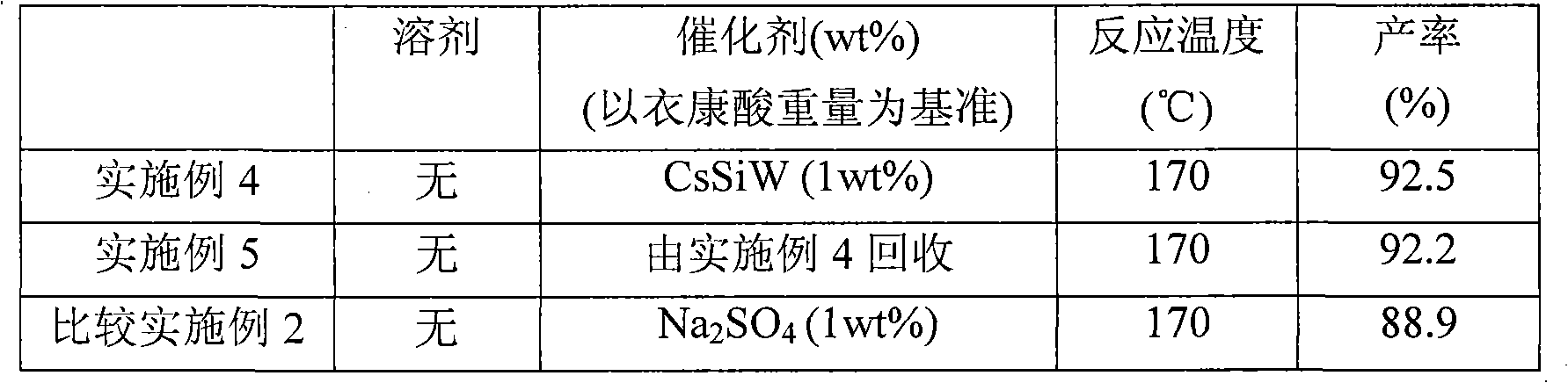
Examples
Embodiment 1
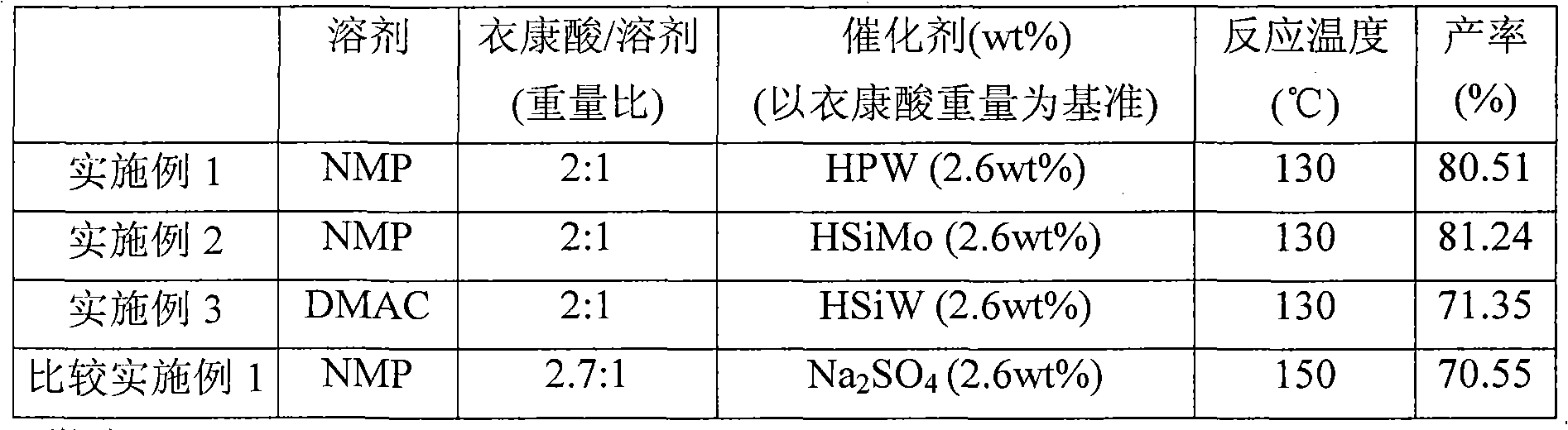
[0026] Add a stirring magnet, 37.9960 grams of itaconic acid, and 1.0020 grams of heteropolyacid catalyst H in a 100ml double-neck round bottom flask 3 PW 12 o 40 , and 19.0180 grams of N-methyl-2-pyrrolidone (N-methyl-2-Pyrrolidone, hereinafter referred to as NMP). After the round bottom bottle was put into an oil pan at 130°C and heated for about 15 minutes, the solution turned yellow-orange and transparent. After the reaction was heated for 30 minutes, the water vapor generated by the reaction was evaporated under the reduced pressure controlled by a water pump at 150-200 torr. After reacting for 4 hours, samples were taken from the round bottom flask, and the yield of citraconic anhydride was analyzed by gas chromatography to be 80.51%. The above reaction can be represented by the following formula:
[0027]
[0028] Please refer to Table 1 for the ratio of reactants and reaction conditions.
Embodiment 2
[0030] Add a stirring magnet, 37.9860 grams of itaconic acid, and 1.0110 grams of heteropolyacid catalyst H in a 100ml double-neck round bottom flask 4 SiMo 12 o 40 , and 18.9960 grams of N-methyl-2 pyrrolidone (N-methyl-2-Pyrrolidone, hereinafter referred to as NMP). After the round bottom bottle was put into an oil pan at 130°C and heated for about 15 minutes, the solution turned yellow-orange and transparent. After the reaction was heated for 30 minutes, the water vapor generated by the reaction was evaporated under the reduced pressure controlled by a water pump at 150-200 torr. After reacting for 4 hours, samples were taken from the round bottom flask, and the yield of citraconic anhydride was analyzed by gas chromatography to be 81.24%. Please refer to Table 1 for the ratio of reactants and reaction conditions.
Embodiment 3
[0032] Add a stirring magnet, 38.0030 grams of itaconic acid, 1.0040 grams of heteropolyacid catalyst H in a 100ml double-neck round bottom flask 4 SiW 12 o 40 , and 19.0080 grams of N, N-dimethylacetamide (N, N-di-methylacetamide, hereinafter referred to as DMAC). After the round bottom bottle was put into an oil pan at 130°C and heated for about 15 minutes, the solution turned yellow-orange and transparent. After the reaction was heated for 30 minutes, the water vapor generated by the reaction was evaporated under the reduced pressure controlled by a water pump at 150-200 torr. After reacting for 4 hours, samples were taken from the round bottom flask, and the yield of citraconic anhydride was analyzed by gas chromatography to be 71.35%. Please refer to Table 1 for the ratio of reactants and reaction conditions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com