Use of anthracene derivatives as anti-infectives
A technology of anti-infective drugs and anthracene derivatives, applied in the field of anthracene derivatives as anti-infective drugs, can solve the problems of unknown effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0037] Hereinafter, the present invention is described in further detail with reference to specific exemplary embodiments, however, this is not meant to limit the scope of the present invention in any way.
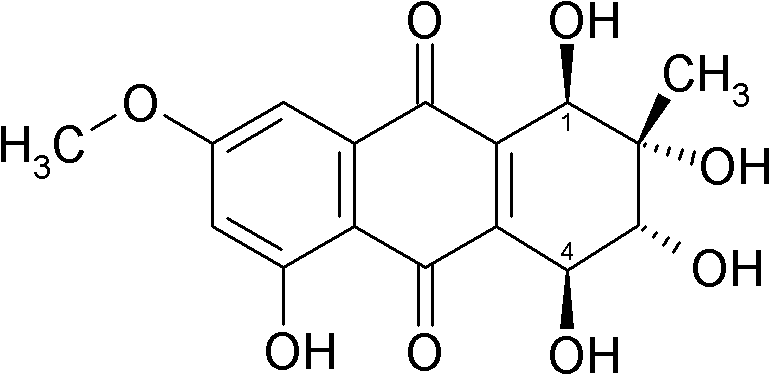
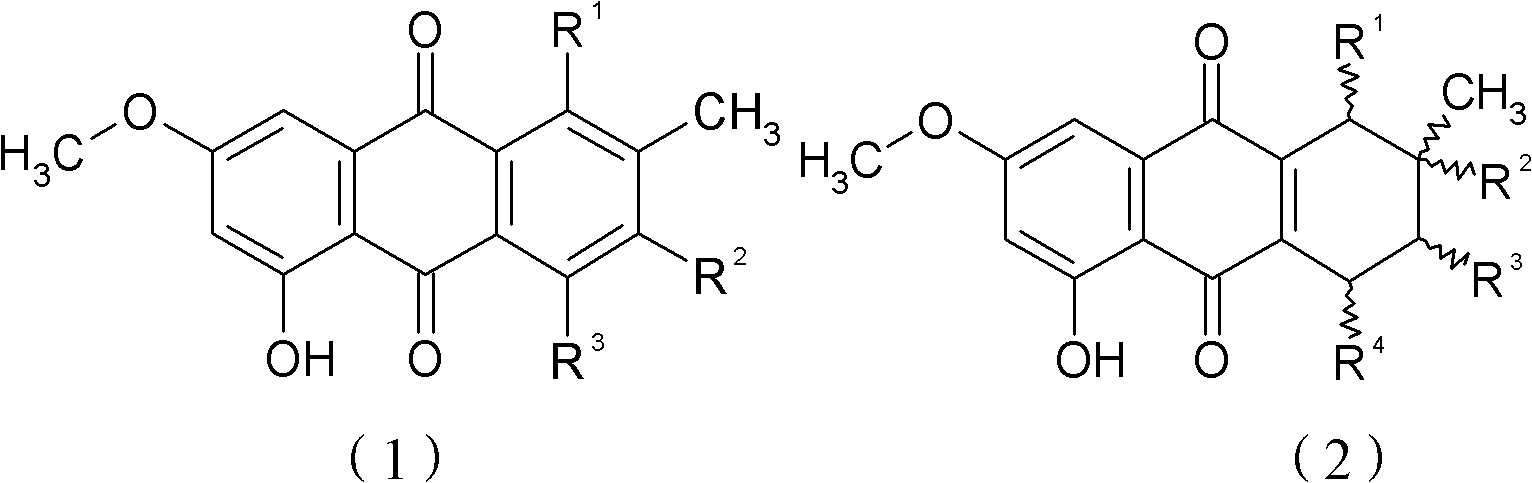
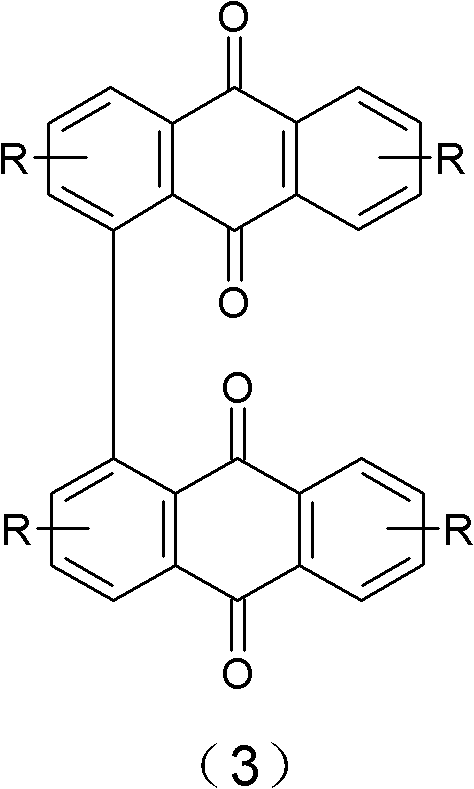
[0038] Compounds of chemical formulas (4) to (8) are obtained by culturing Puccinia glomerulus to obtain compounds (5) to (7) and Nigersporium to obtain compounds (4) and (8), followed by extraction.
[0039] separate
[0040] a) Puccinia globosa
[0041] Endophytic fungi were isolated from fresh, healthy stems of Mint lipelum (Peppermint). The surface of the stem was sterilized with 70% ethanol for 1 min, followed by rinsing with sterile water to remove the ethanol. To distinguish endophytic fungi from any remaining epiphytic fungi, stem surface imprints were obtained on organic malt extract agar. A small sample of internal tissue is sterilely sectioned and pressed onto agar plates containing an antibiotic to inhibit bacterial growth. The composition of the isolation...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com