Methods and compositions for diagnostic assays for measuring cell mediated immune response
一种免疫应答、细胞的技术,应用在诊断试验领域,达到降低试验成本、增加特异性的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
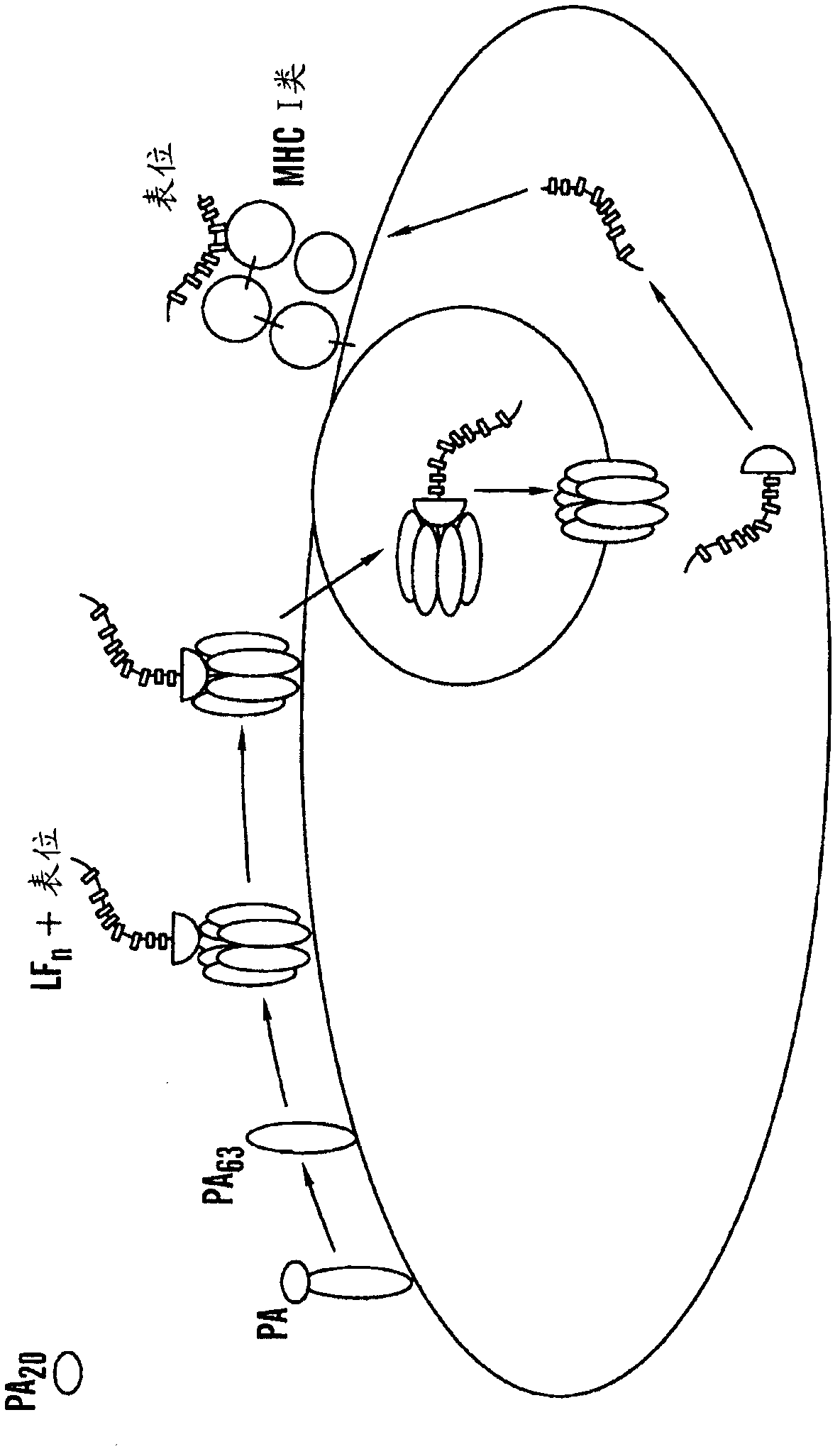
[0380] 1. A method of measuring a cell-mediated immune response (CMI) to a target antigen in a subject, said method comprising the steps of:
[0381] a. Incubate a biological sample from said subject with at least one fusion polypeptide comprising
[0382] A part of the LF polypeptide that lacks LF enzyme activity but is sufficient to facilitate transmembrane delivery of said fusion polypeptide to cells,
[0383] said portion is fused to a target antigen polypeptide or fragment thereof, wherein said biological sample comprises cells of the immune system that release at least one cytokine in response to the antigen;
[0384] b. measuring the level of at least one cytokine released in said biological sample; and
[0385] c. comparing the level of said cytokine in said biological sample to a reference level of the same cytokine,
[0386] wherein an increase in the level of said cytokine in said biological sample from a subject relative to a reference level of cytokine is indica...
Embodiment 1
[0540] IFN-γ ELISA protocol. As an example, a 1 plate (96 well) protocol is described below, with calculations based on 100 wells.
[0541] Coating: 10 μl of capture antibody (stock solution 1 mg / ml) was added to 10 ml of coating solution to dilute the capture antibody to 1 ug / ml. 100 μl of capture antibody at 1 μg / ml was added to each well of a 96-well plate and incubated overnight at 4°C. After this incubation at 4°C, the capture antibody is aspirated from the wells and the wells are washed at least 3 times, preferably 5 times with 250 μl PBST / well. After the washing step, the wells were blocked with 250 μl of blocking buffer per well for 2 hours at 37°C.
[0542] Wash step: Blocking solution was aspirated from the wells, followed by the addition of 250 μl PBST / well. This process is repeated at least 3 times, preferably 5 times.
[0543] Sampling procedure: 100 μl of IFN-γ for each standard curve dilution (ie from 300 pg / ml to 4.7 pg / ml, see “Standard Curve Preparation (...
Embodiment 2
[0551] Confirm the TB smear test using an in vitro CMI response test. Samples from 13 patients identified as positive for TB in the TB smear test were assayed for CMI response to TB using the diagnostic assay described herein. The 13 samples were also determined to be positive for TB using a culture assay. After incubation with LFn-TB antigen, INF-γ levels were determined using ELISA. The results are shown in Tables 3 and 4.
[0552] Table 3 shows an example of the results of the CMI assay disclosed herein as a diagnostic and prognostic identification of subjects with TB in which INF-γ levels were measured as follows: Using an ELISA assay, INF-γ protein level.
[0553]
[0554] Samples in bold were identified as TB positive by TB smear test and TB culture test. In this example, the assay was performed as follows: using reblock plates, Lot No. 20081217, GMP INF-γ (Lot No. 20081001) for standard curve - 1 mg / ml, detection Ab Pierce, Lot No. IJ116994. HRP: Biosource, Lot N...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com