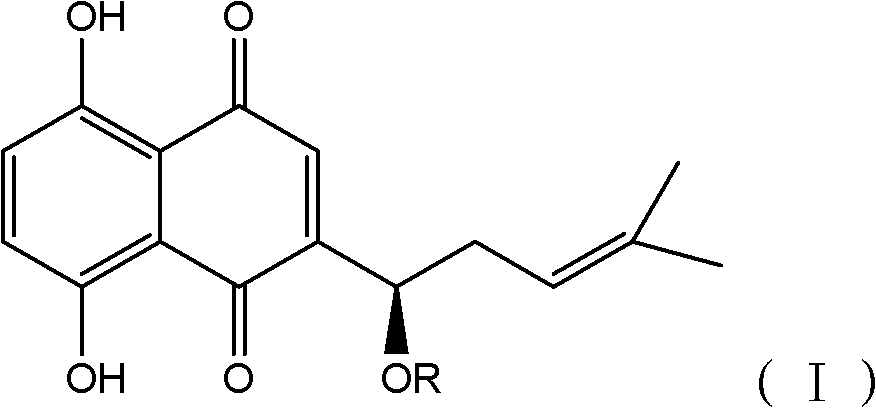
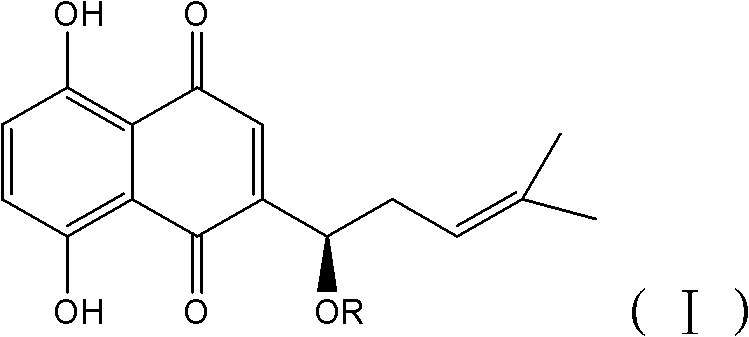
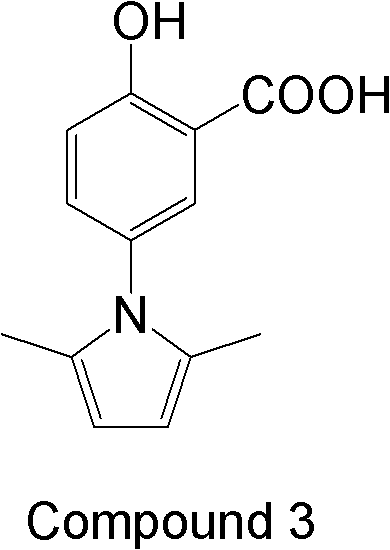
Application of alkannin glucoside to preparation of pyruvate kinase inhibitor
A technology of pyruvate kinase and shikonin, applied in medical preparations containing active ingredients, organic active ingredients, drug combinations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] The present invention is further described below in conjunction with specific embodiment, but protection scope of the present invention is not limited thereto:
[0029] Experimental Materials:
[0030] Shikonin glycosides are synthesized by themselves in the present invention, and the synthesis method is as follows:
[0031] Under the protection of nitrogen, add activated 4A molecular sieve to trichloroacetimide ester containing glycosyl or acetyl-substituted glycosyl and 1 to 2 times the mole of shikonin, and dissolve dichloro The boron trifluoride ether solution of 0.1 times the molar amount of methane was added dropwise in about 30 minutes, and the reaction was continued for 1 hour, and the reaction process was tracked by TLC. After the reaction, triethylamine was added to neutralize the boron trifluoride in the reaction solution, and then the solution was changed from dark blue to red with acetic acid, filtered, the filtrate was spin-dried, and separated by column ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com