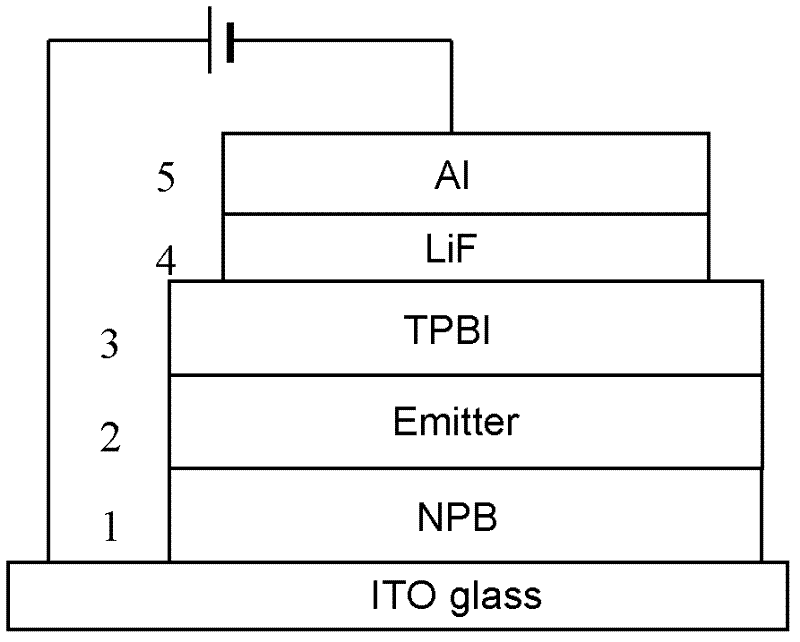
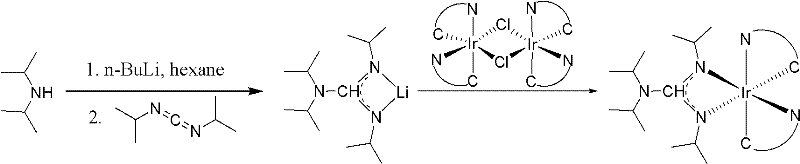
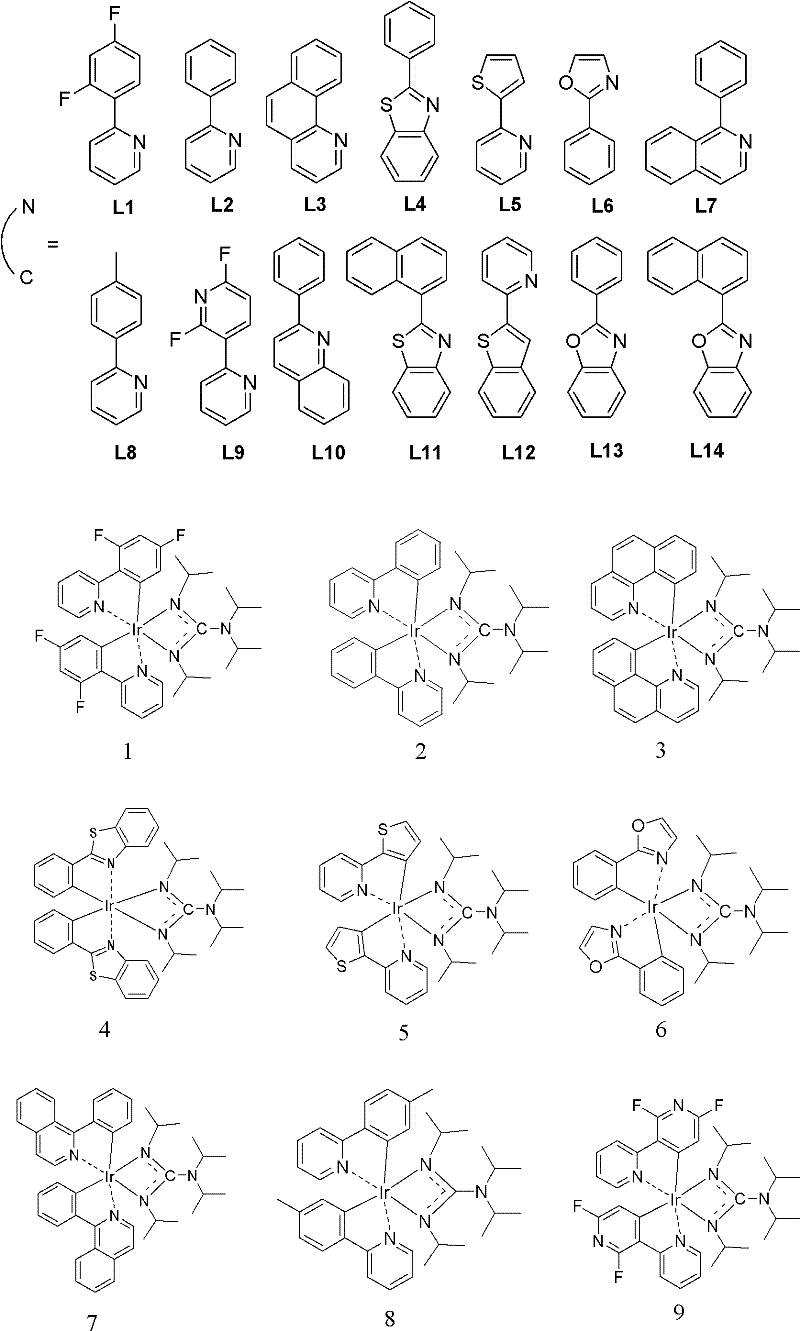
Iridium complexes containing guanidine group and application of iridium complexes to preparation of electroluminescent devices
A technology of iridium complexes and luminescence, which is applied in the direction of electric solid devices, compounds containing elements of group 8/9/10/18 of the periodic table, electrical components, etc., can solve the problem of high cost, affecting product quality and commercial competitiveness , Poor carrier transport ability and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1: the synthesis of compound 1
[0016] In a 50 mL round bottom flask at -78°C under nitrogen protection, n-BuLi (0.2 mL x 2.5M) was added dropwise to a solution of diisopropylamine (50 mg, 0.5 mmol) in n-hexane (15 mL). After stirring for half an hour, N,N'-diisopropylcarbodiimide (63 mg, 0.5 mmol) was added dropwise to the mixture. After the colorless solution was stirred for another 30 minutes, it was added dropwise to a n-hexane solution (20 mL) of L1 bridged iridium complex (0.25 mmol, 303 mg). After the addition, the temperature was raised to 80° C. and stirred for 8 hours. The reaction solution was cooled to room temperature, the solvent was evaporated under reduced pressure, and the crude product was washed three times with ether (20 mL) to obtain compound 1 (212 mg). Mass spectrometry proves that its molecular weight is 798.9 (C 35 h 40 f 4 IrN 5 ). The elemental analysis results are: C, 52.57; H, 5.08; N, 8.54 (theoretical values: C, 52.62; H,...
Embodiment 2
[0017] Embodiment 2: the synthesis of compound 2
[0018] In a 50 mL round bottom flask at -78°C under nitrogen protection, n-BuLi (0.2 mL x 2.5M) was added dropwise to a solution of diisopropylamine (50 mg, 0.5 mmol) in n-hexane (15 mL). After stirring for half an hour, N,N'-diisopropylcarbodiimide (63 mg, 0.5 mmol) was added dropwise to the mixture. After the colorless solution was stirred for another 30 minutes, it was added dropwise to a n-hexane solution (20 mL) of L2 bridged iridium complex (0.25 mmol, 267 mg), and after the addition, the temperature was raised to 80° C. and stirred for 8 hours. The reaction solution was cooled to room temperature, the solvent was distilled off under reduced pressure, and the crude product was repeatedly washed three times with ether (20mL) to obtain compound 2 (133mg). Mass spectrometry analysis proved that its molecular weight was 726.99 (C 35 h 44 IrN 5 ). The elemental analysis results are: C, 57.83; H, 5.97; N, 9.65 (theoretical...
Embodiment 3
[0019] Embodiment 3: the synthesis of compound 3
[0020] In a 50 ml round bottom flask at -78°C under nitrogen protection, n-BuLi (0.2 mL x 2.5M) was added dropwise to diisopropylamine (50 mg, 0.5 mmol) in n-hexane (15 mL). After stirring for half an hour, N,N'-diisopropylcarbodiimide (63 mg, 0.5 mmol) was added dropwise to the mixture. After the colorless solution was stirred for another 30 minutes, it was added dropwise to a n-hexane solution (20 mL) of L3 bridged iridium complex (0.25 mmol, 292 mg). After the addition, the temperature was raised to 80° C. and stirred for 8 hours. The reaction solution was cooled to room temperature, the solvent was distilled off under reduced pressure, and the crude product was repeatedly washed three times with ether (20mL) to obtain compound 3 (155mg). Mass spectrometry analysis proved that its molecular weight was 774.99 (C 39 h 44 IrN 5 ). The elemental analysis results are: C, 60.24; H, 5.78; N, 8.92 (theoretical values: C, 60.44;...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com