Method for efficiently producing pro-TGase through fermentation
A technology for producing transglutaminase enzymes and strains is applied in the field of high-efficiency fermentation and production of transglutaminase proenzymes, which can solve problems such as low secretion of transglutaminase proenzymes, and achieve good application prospects. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
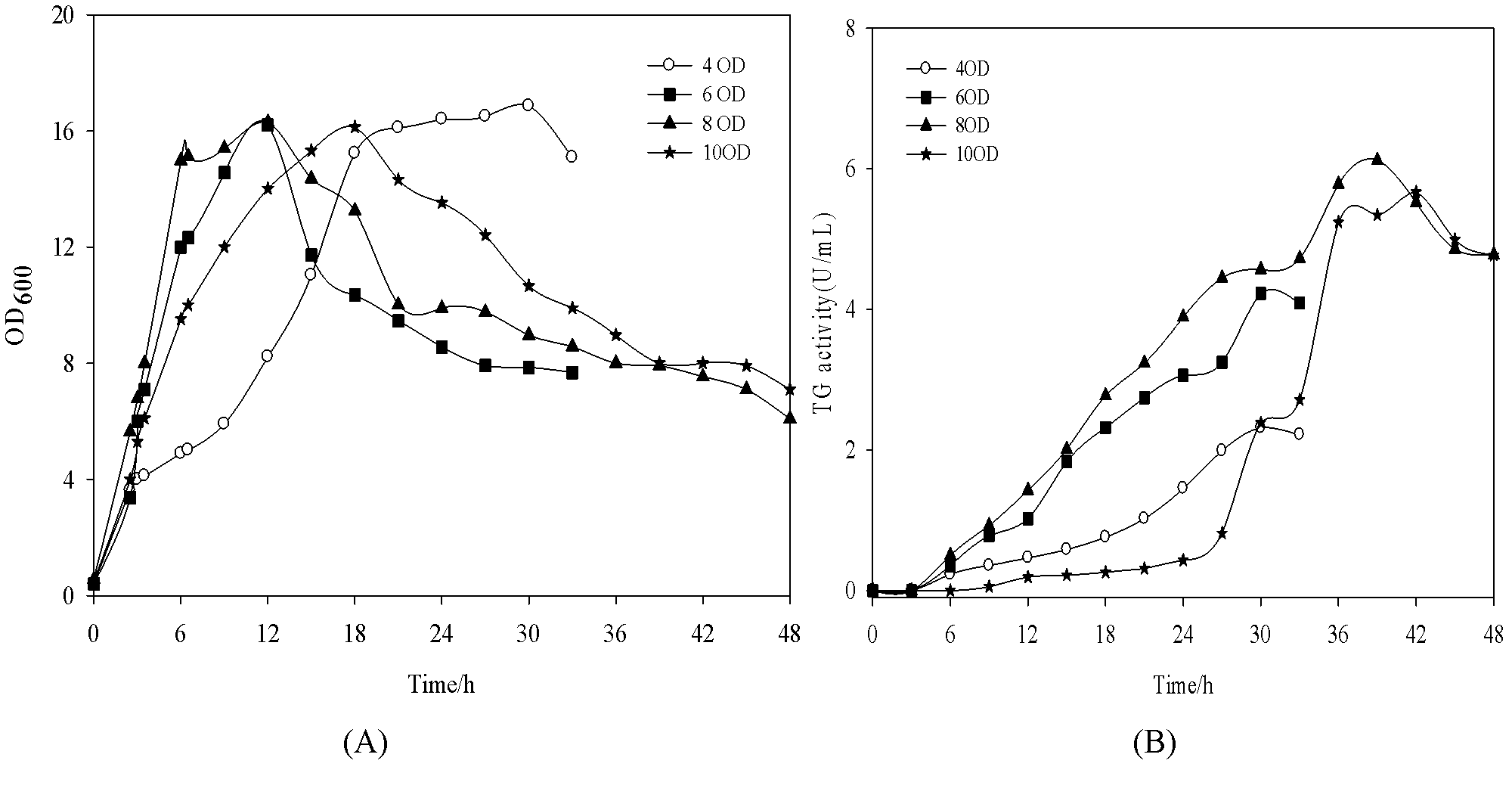
[0017] The logarithmic growth phase controls the temperature at 37°C, when the bacteria grow to OD 600 At 4, 6, 8, and 10 o'clock respectively, the temperature was lowered to 20°C and IPTG with a final concentration of 0.4mM was added to promote the high-efficiency expression of pro-TGase. Until the end of fermentation, the induced OD 600 =8, the highest enzyme activity was 6.22U / mL, and the production intensity was 0.16U / mL / h ( figure 1 ).
Embodiment 2
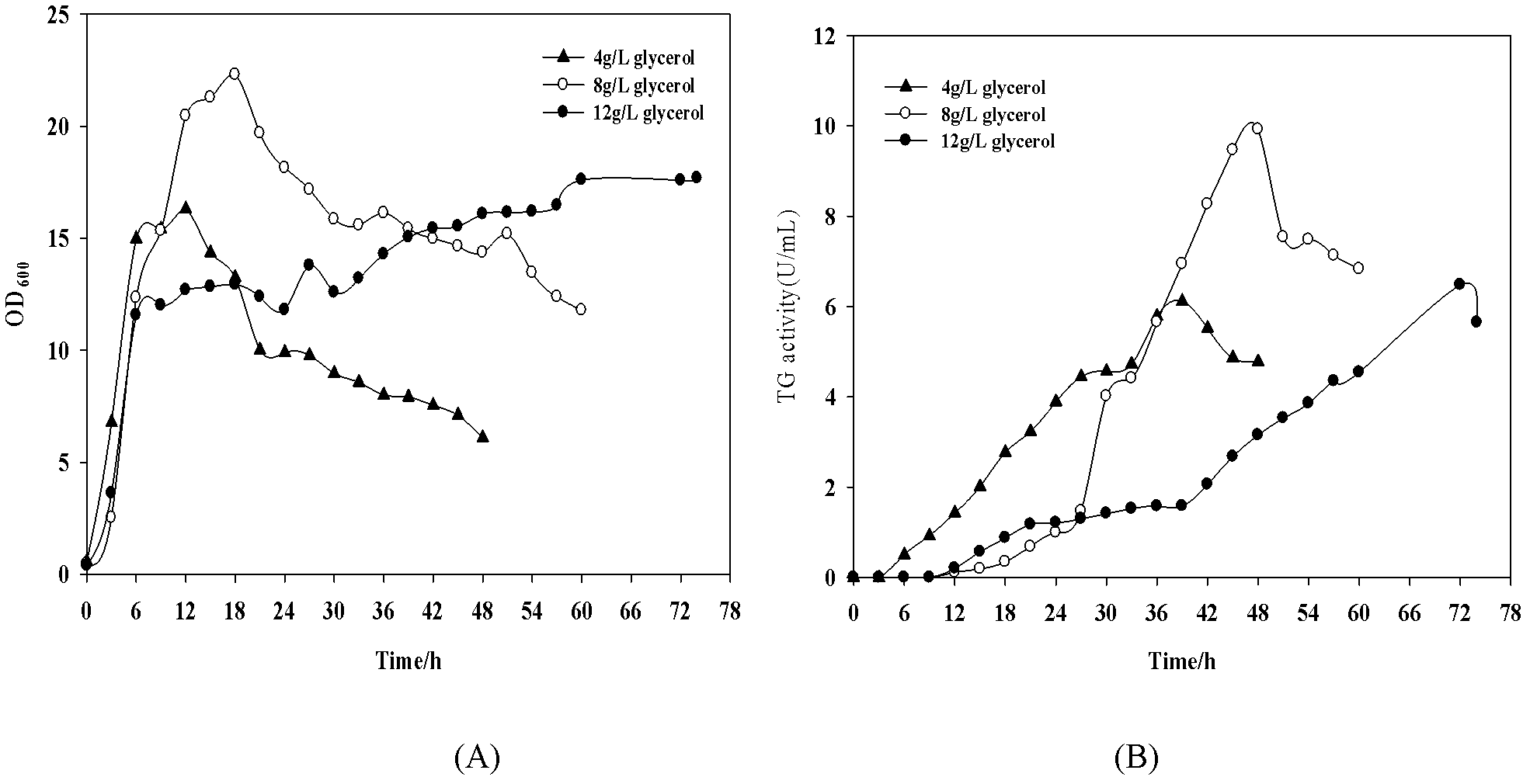
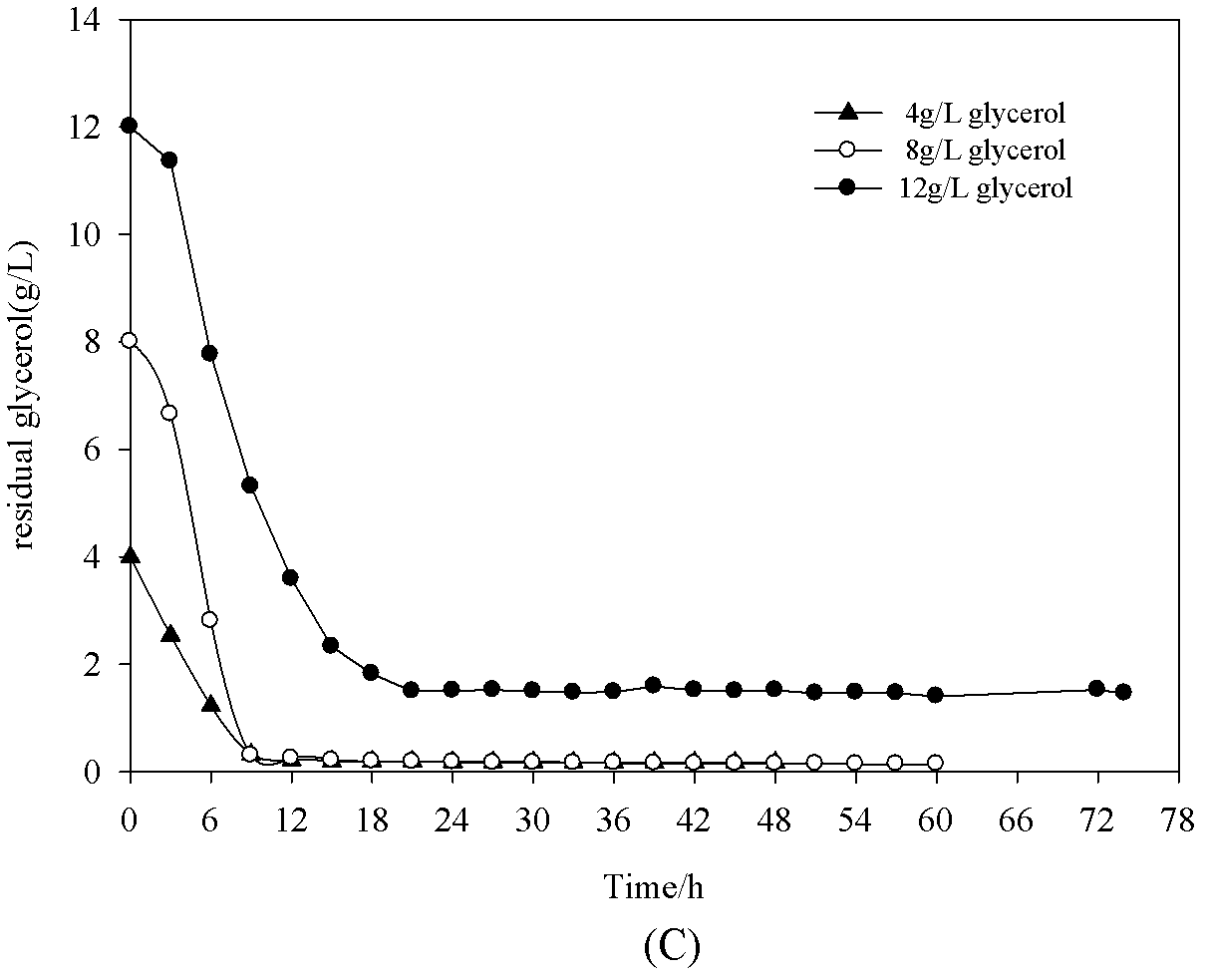
[0019] Change the initial glycerol concentration to 4g / L, 8g / L and 12g / L respectively, at OD 600 = Induction at 8 hours. Culture condition is the same as example 1, until fermentation finishes ( figure 2 ). When the initial glycerol concentration was 8g / L, the highest enzyme activity was 9.92U / mL, and the production intensity was 021U / mL / h, which was 31.25% higher than the previous production intensity ( image 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com