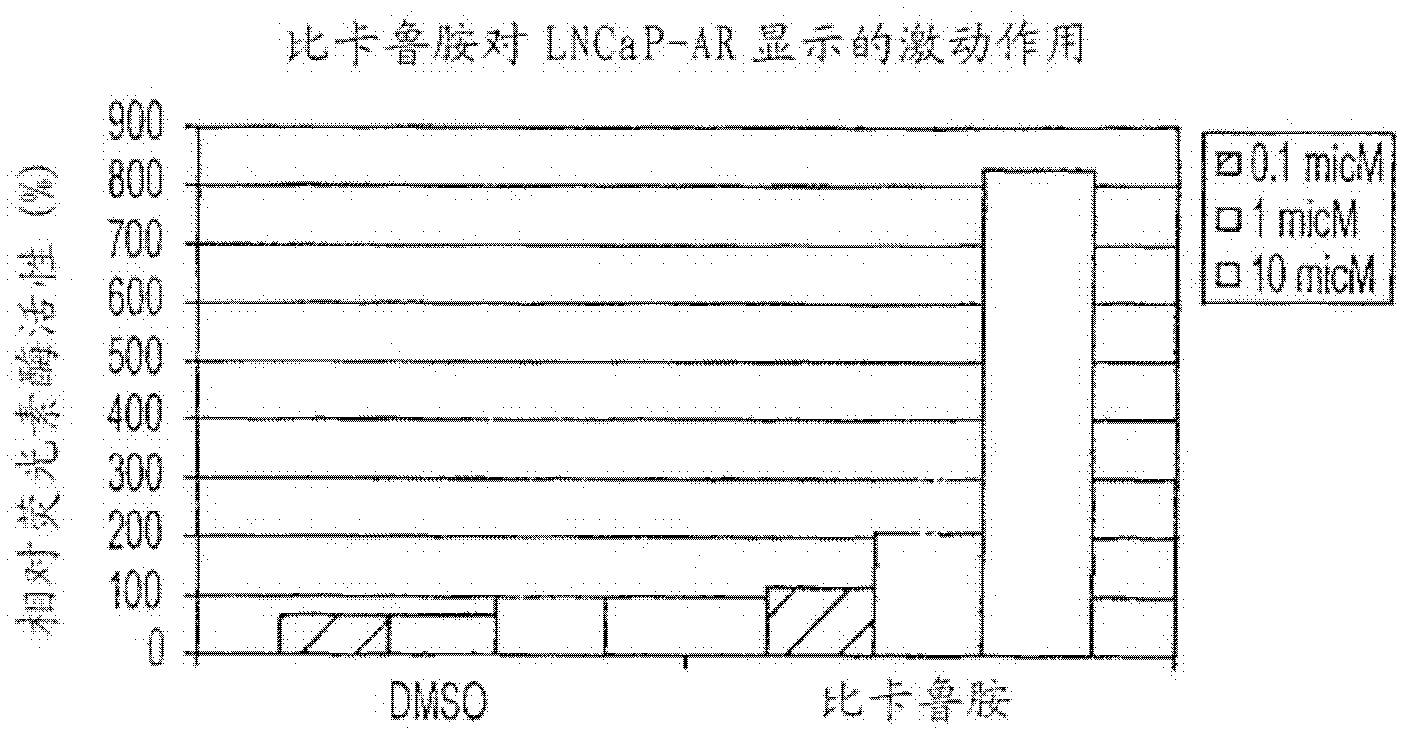
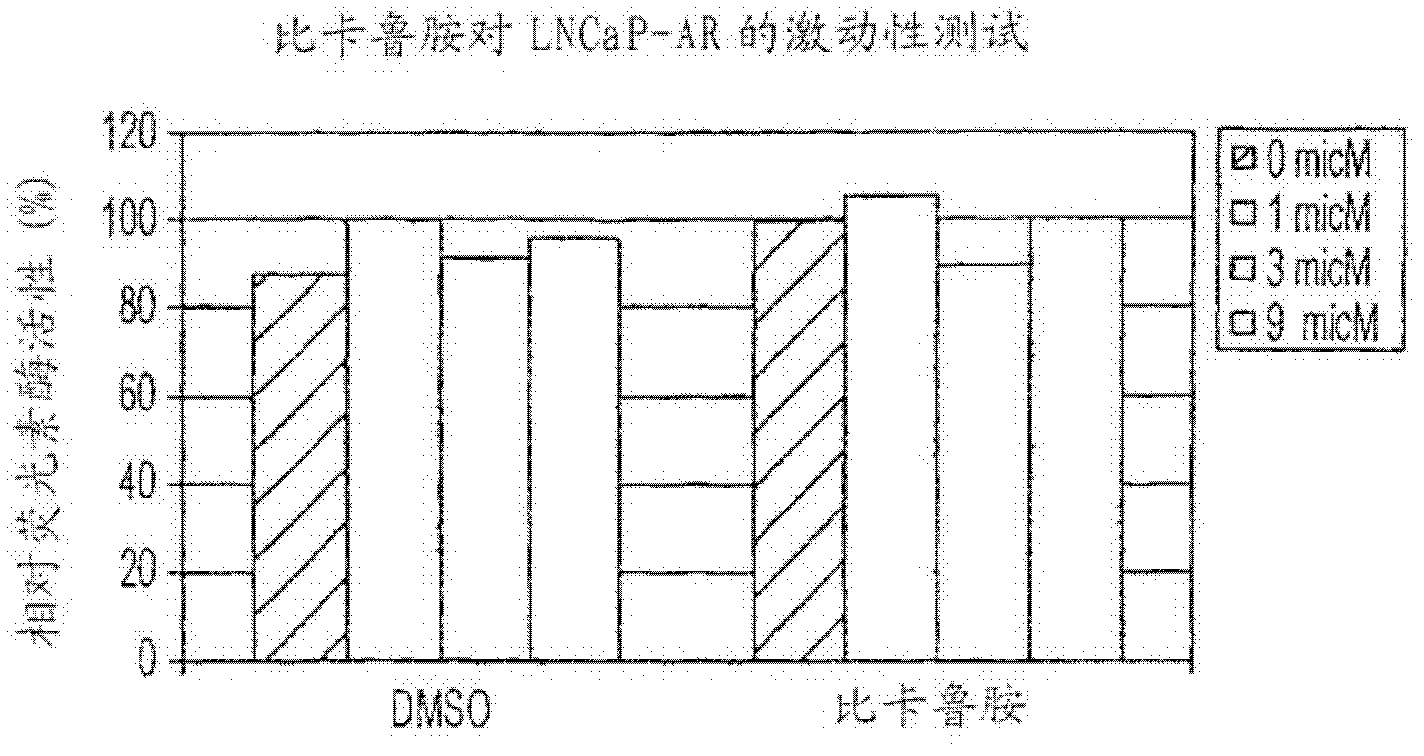
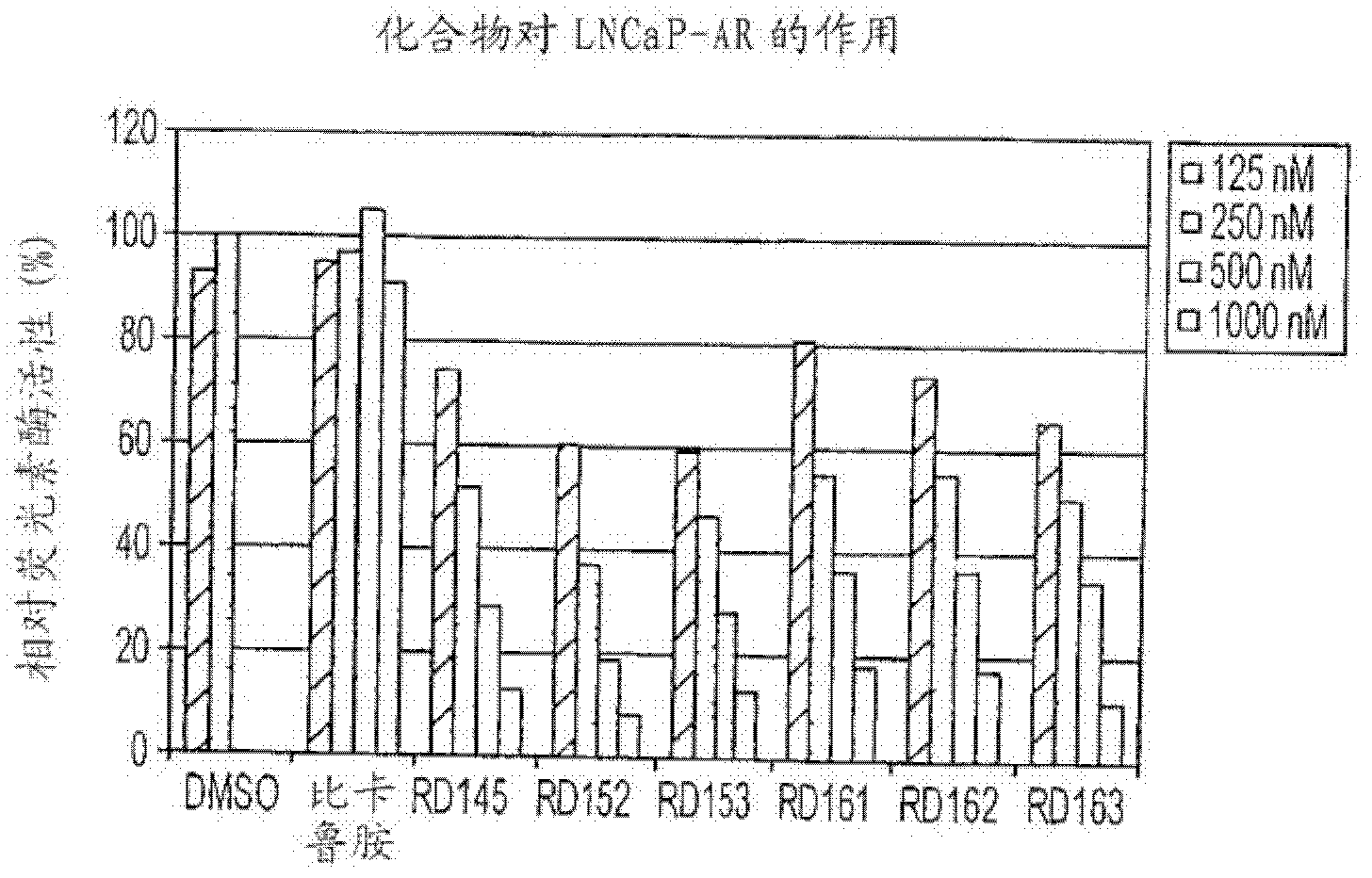
Diarylhydantoin compounds
一种化合物、氨基甲酰基的技术,应用在二芳基乙内酰脲化合物领域,能够解决耗费时间等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0131] The present invention includes compounds having the formula:
[0132]
[0133] Wherein X is selected from trifluoromethyl and iodine, wherein W is selected from O and NR5, wherein R5 is selected from the following groups: H, methyl and
[0134]
[0135] wherein D is S or O, and E is N or O, and G is alkyl, aryl, substituted alkyl or substituted aryl; or D is S or O and E-G together are C1-C4 lower alkyl,
[0136] wherein R1 and R2 together contain 8 or less carbon atoms and are selected from the group consisting of alkyl, substituted alkyl including haloalkyl, and, together with the carbon atoms to which they are attached, cycloalkyl or substituted cycloalkyl,
[0137] Wherein R3 is selected from the following groups: hydrogen, halogen, methyl, C1-C4 alkoxy, formyl, haloacetoxy, trifluoromethyl, cyano, nitro, hydroxyl, phenyl, amino, methyl Carbamoyl, methoxycarbonyl, acetylamino, methylsulfonylamino, methylsulfonyl, 4-methylsulfonyl-1-piperazinyl, piperazinyl, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com