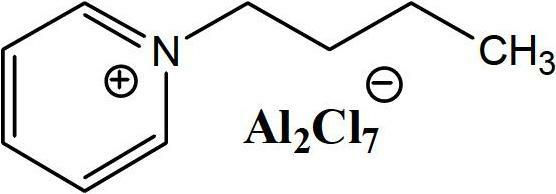
Hydroisomerization and selective hydrogenation of feedstock in ionic liquid-catalyzed alkylation
一种酸性离子液体、烷基化物的技术,应用在烷基化领域,能够解决烷基化效率降低、不适宜聚合物副产物等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
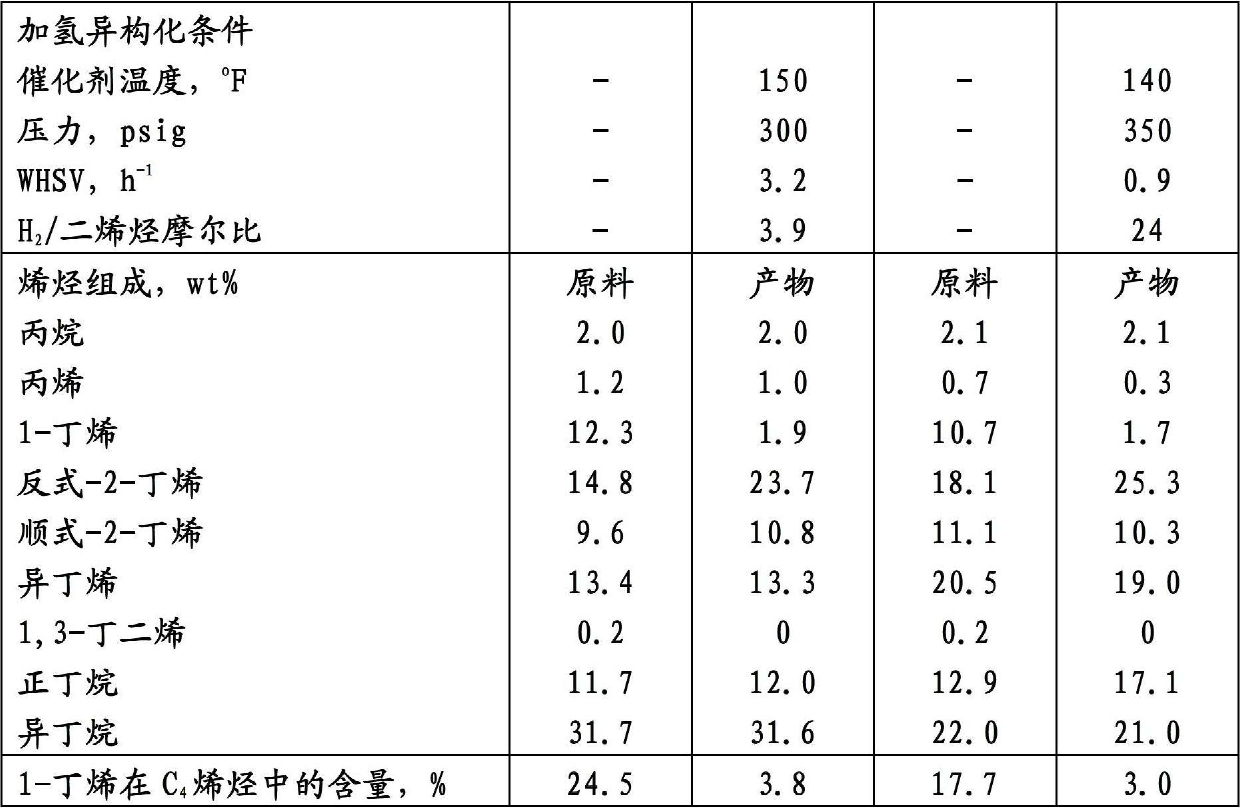
[0049] C 4 Hydroisomerization of Olefin Feedstocks
[0050] Table 1 shows the refinery C 4 Olefin feedstock is using 0.5wt%Pd / Al 2 o 3 Composition before and after catalyst hydroisomerization. It demonstrates the conversion of 1-butene to 2-butene and complete saturation of 1,3-butene by the hydroisomerization process. The 1-butene in the hydroisomerization product is at C4 Concentrations in olefins are below 5%, with little or no loss of olefins.
[0051] Table 1.C 4 Composition of olefin streams before and after hydroisomerization
[0052]
Embodiment 2
[0054] C 3 and C 4 Hydroisomerization of Olefin Feedstocks
[0055] Table 2 shows the propylene-rich refinery C 4 The olefin feed stream is using 0.5wt% Pd / Al 2 o 3 Composition of the catalyst before and after hydroisomerization. It demonstrates the conversion of 1-butene to 2-butene and complete saturation of 1,3-butene by this hydroisomerization. C 4 1-Butene among the olefins is isomerized from 19.9% in the feed to about 6% in the product.
[0056] Table 2.C 4 Composition of olefin streams before and after hydroisomerization
[0057]
[0058]
Embodiment 3
[0060] Refinery C. 5 Hydroisomerization of Olefin Feedstocks
[0061] Table 3 shows that at 0.5wt% Pd / Al 2 o 3 C before and after hydroisomerization on the catalyst 5 Olefin composition. 1-Pentene is isomerized to 2-Pentene with over 80% conversion over this catalyst. 3-Methyl-1-butene and 2-methyl-1-butene were converted to 2-methyl-2-butene at about 50% conversion.
[0062] Table 3.C 5 Composition of olefin streams before and after hydroisomerization
[0063] Hydroisomerization Conditions
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| octane number | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com