Medical application of 1,2,3,4,6-O-pentagalloylglucose and composition thereof in preparation of demulcent medicaments
A technology of pentagalloyl glucose and its composition, which is applied in the field of preparing analgesic drugs, and can solve problems such as abnormal and allergic reactions, side effects that are difficult to eliminate, and adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
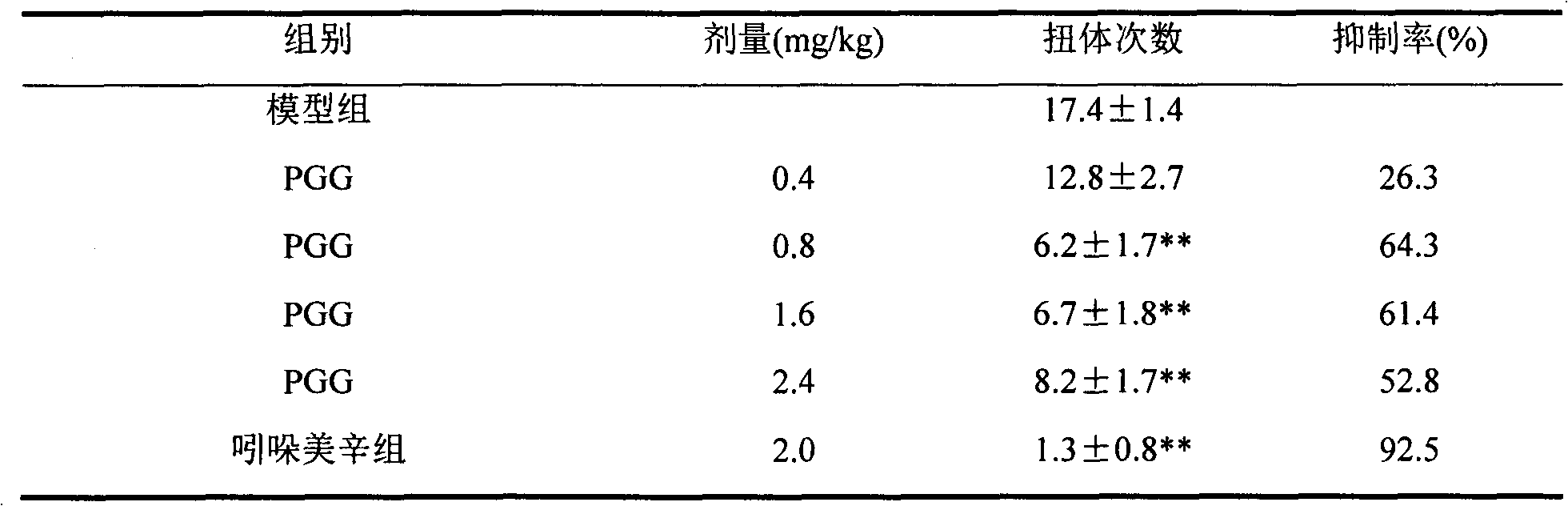
[0006] Inhibitory effect of PGG on visceral pain.
[0007] Blank control: 5% ethanol solution
[0008] Positive control: Indomethacin (2mg / kg)
[0009] Sample: sample PGG, after dissolving in ethanol, dilute to 5% ethanol solution, and prepare 2.4mg / kg, 1.6mg / kg, 0.8mg / kg, 0.4mg / kg solutions respectively. Indomethacin was made into a 2mg / kg solution with 5% ethanol solution.
[0010] Experimental method: 50 ICR mice, weighing 20±2g, were randomly divided into 5 groups, 10 mice in each group, and the drugs in each group were administered by intragastric administration. Concave, hind limbs straight, buttocks raised as indicators, record the number of writhing times of mice in each group within 15 minutes, and calculate the percentage of analgesic inhibition. Analgesic percentage (%)=(average number of writhing in the control group−average number of writhing in the treatment group) / average number of writhing in the control group×100%.
[0011] Experimental results: see Table ...
Embodiment 2
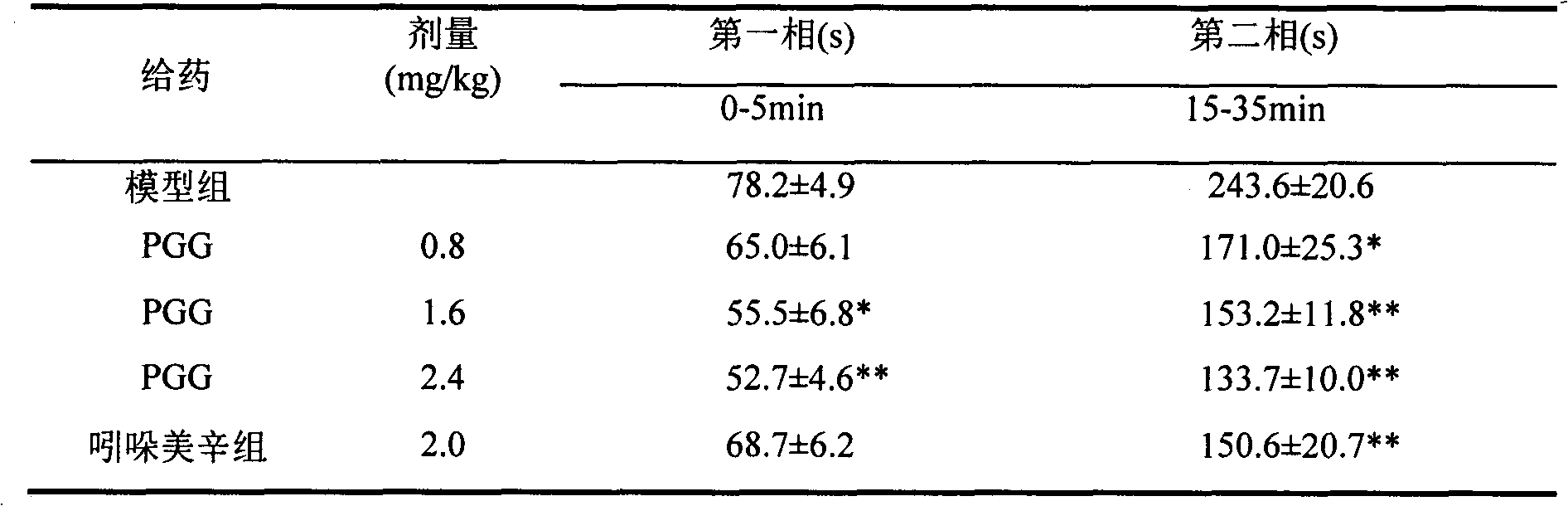
[0017] Effects of PGG on central and peripheral pain.
[0018] Blank control: 5% ethanol
[0019] Positive control: Indomethacin (2mg / kg)
[0020] Sample: sample PGG, after dissolving in ethanol, dilute to 5% ethanol solution, and prepare 2.4mg / kg, 1.6mg / kg, 0.8mg / kg solutions respectively. Indomethacin was made into a 2mg / kg solution with 5% ethanol solution.
[0021] experimental method:
[0022] Experimental method: 50 ICR mice, weighing 20±2g, were randomly divided into 5 groups, each group was administered by intragastric administration, and 20 μl of 2.5% formaldehyde solution was injected into the right hind foot of the mice 1 hour after the administration, and each time was recorded. The paw licking time of mice in the first phase (0-5min) and the second phase (15-35min).
[0023] Experimental results: see Table 2
[0024] Table 2 Effect of PGG on formaldehyde-induced pain mice
[0025]
[0026] *P<0.05, **P<0.01 compared with model group
[0027] Formaldeh...
Embodiment 3
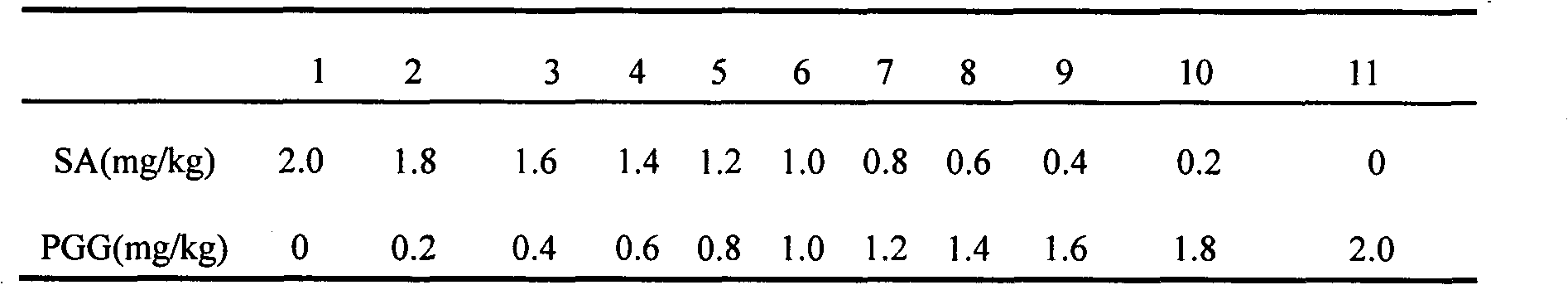
[0030] Analgesic effect of PGG and SA combined in specific ratios.
[0031] Blank control: 5% ethanol solution
[0032] Positive control: Indomethacin (2mg / kg)
[0033] Sample: According to the baseline equal ratio increase and decrease method, under the premise of a constant total amount, take SA / PGG (3 / 2) as the baseline, expand to both sides, keep a distance, and finally expand to the pole, and divide it into several variable groups in the middle. During this period, the SA content decreased by 20%, and the PGG content increased by 20%. Conversely, the PGG content decreased by 20%, and the SA content increased by 20%, expanding to both sides.
[0034] Table 3 Dosage groups set according to the baseline proportional increase and decrease method
[0035]
[0036] Select (2:0, 4:1, 7:3, 3:2, 1:1, 2:3, 1:4, 0:2) from Table 3 (1, 3, 4, 5, 6, 7, 9, 11) groups.
[0037] Experimental method: 100 ICR mice, weighing 20±2g, were randomly divided into 10 groups. The drugs in eac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com