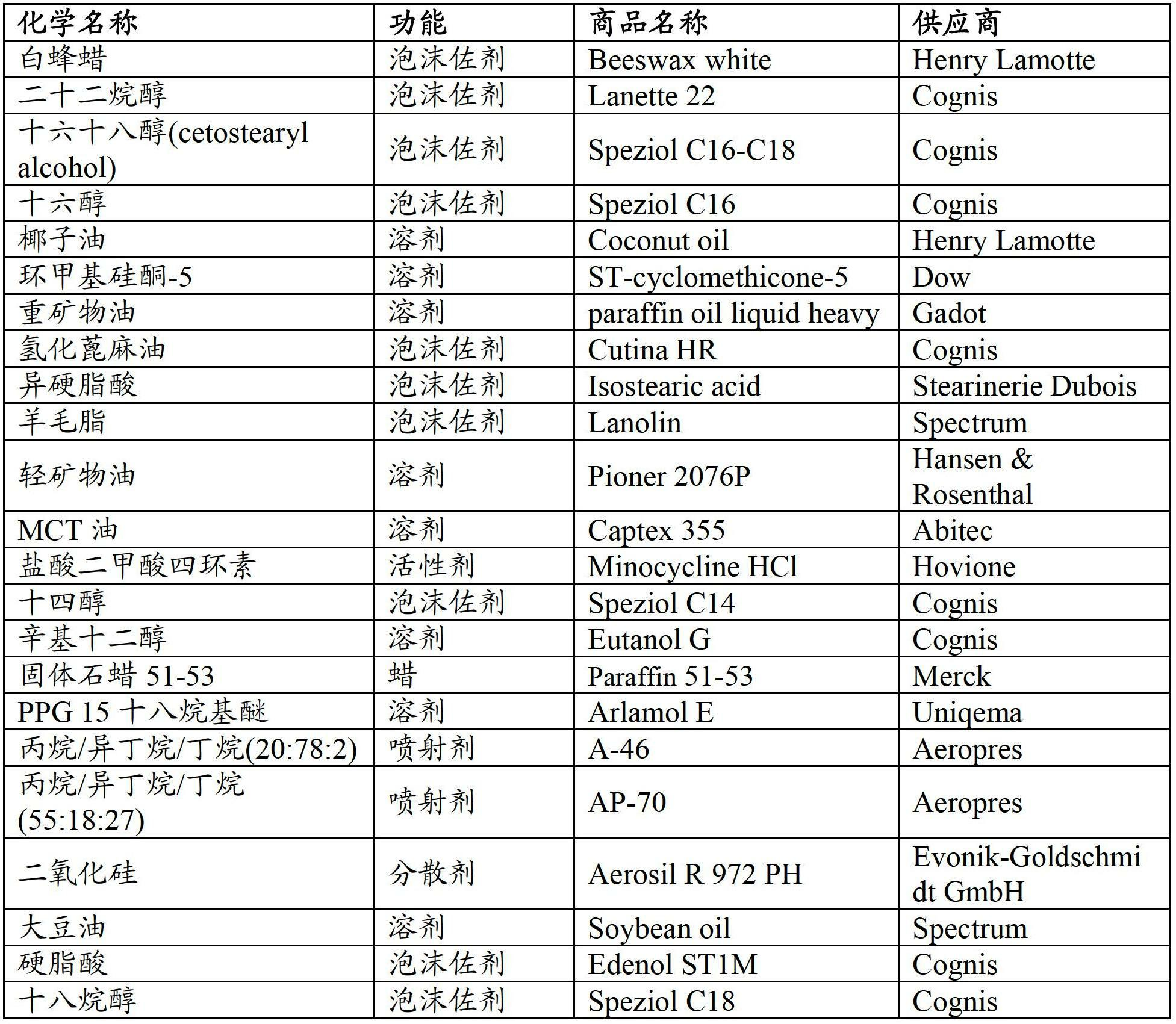
Topical tetracycline compositions
A kind of composition, technology of tetracycline, applied in the field of topical tetracycline composition, can solve problems such as troublesome, not having
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0223] Example 1 - General manufacturing steps for the gel
[0224] The following steps were used to produce the gel samples described in the following examples, where only the steps relevant to each formulation were performed depending on the type and nature of the ingredients used.
[0225] Step 1: Heat the hydrophobic oil to 60 to 70°C.
[0226] Step 2: Add fatty alcohol (if present), fatty acid (if present), wax (if present) to hydrophobic oil and mix formulation until fully melted.
[0227] Step 3: Cool the formulation to 30 to 40°C, add the tetracycline antibiotic and mix the formulation until homogeneity is obtained.
[0228] Step 4: Cool the formulation to room temperature with stirring and pack into suitable containers.
[0229] As will be understood by those skilled in the art, the experiments are only briefly set forth below by way of non-limiting example.
[0230] Viscosity was measured with a Brookfield LVDV-II+PRO with spindle SC4-25 at ambient temperature and...
Embodiment 2
[0232] Example 2 - Low Viscosity Gel Formulation
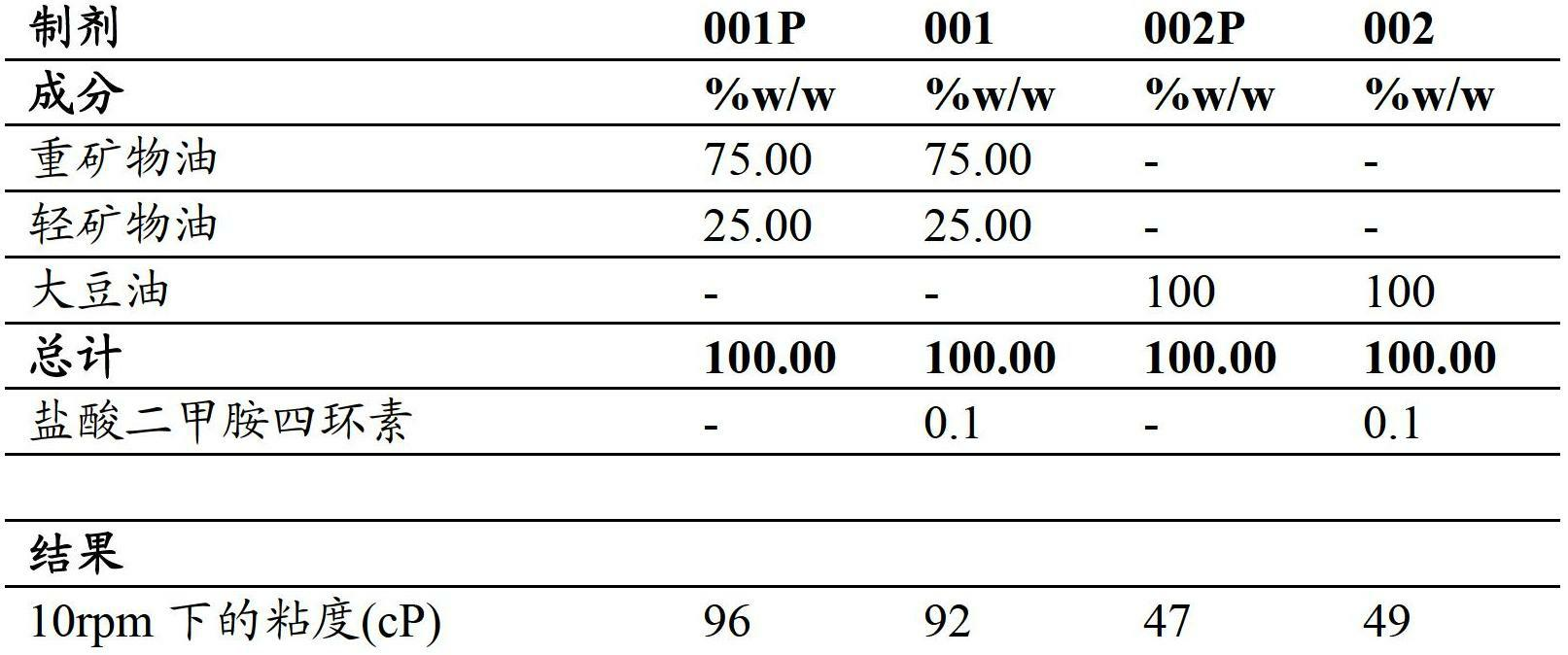
[0233] The different hydrophobic oils suitable for use in topical pharmaceutical compositions are generally liquid oils with low viscosity. As described in Table 2, when these oils are used as such for topical delivery of active agents, they especially have two undesirable properties: (1) Due to their low viscosity, they tend to drip and flow easily, thus being easy on the patient. Applied on the skin, (2) they have poor suspension properties, resulting in rapid sedimentation of the insoluble active ingredient (API).
[0234] Table 2 - Low Viscosity Oily Formulations
[0235]
[0236] As shown in formulations 001P and 002P, the mixture of mineral oil and soybean oil has low viscosity. Formulations 001 and 002, show that after addition of minocycline hydrochloride, the viscosity of the formulation remains unchanged and the active ingredient is deposited.
Embodiment 3
[0237] Example 3 - Mineral Oil Formulations with Improved Viscosity
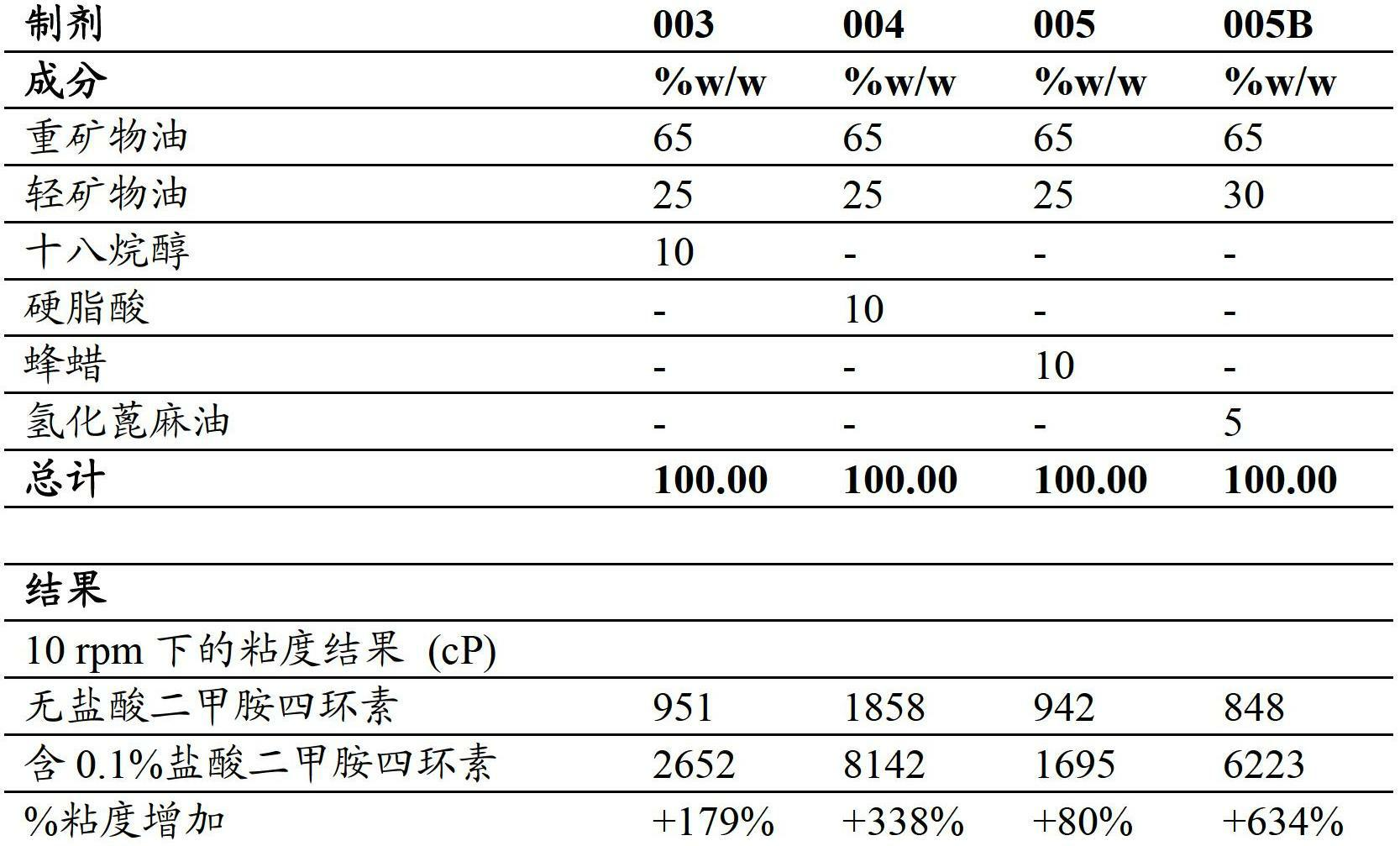
[0238] The effect of tetracycline in combination with fatty alcohols, fatty acids and waxes on formulation viscosity was evaluated as described in Table 3a. Formulations containing mixtures of mineral oil and fatty alcohols, fatty acids or waxes were prepared and their viscosities were measured before and after the addition of tetracycline, minocycline hydrochloride. Table 3a below shows the results for the viscosity of the formulations before and after the addition of tetracycline, and the percentage increase in viscosity due to the addition of the active ingredient.
[0239] Table 3a - Combinations of Tetracyclines with Fatty Alcohols, Fatty Acids and Waxes
[0240]
[0241] Quite surprisingly, it was found that the addition of minocycline hydrochloride to mineral oil based formulations 003 to 005B resulted in a very large increase in viscosity despite using a very low amount of minocycline hydrochlori...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
| Minimum inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com