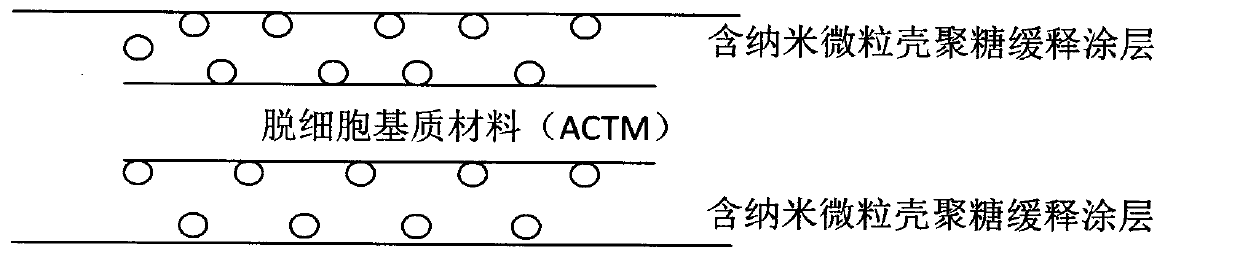
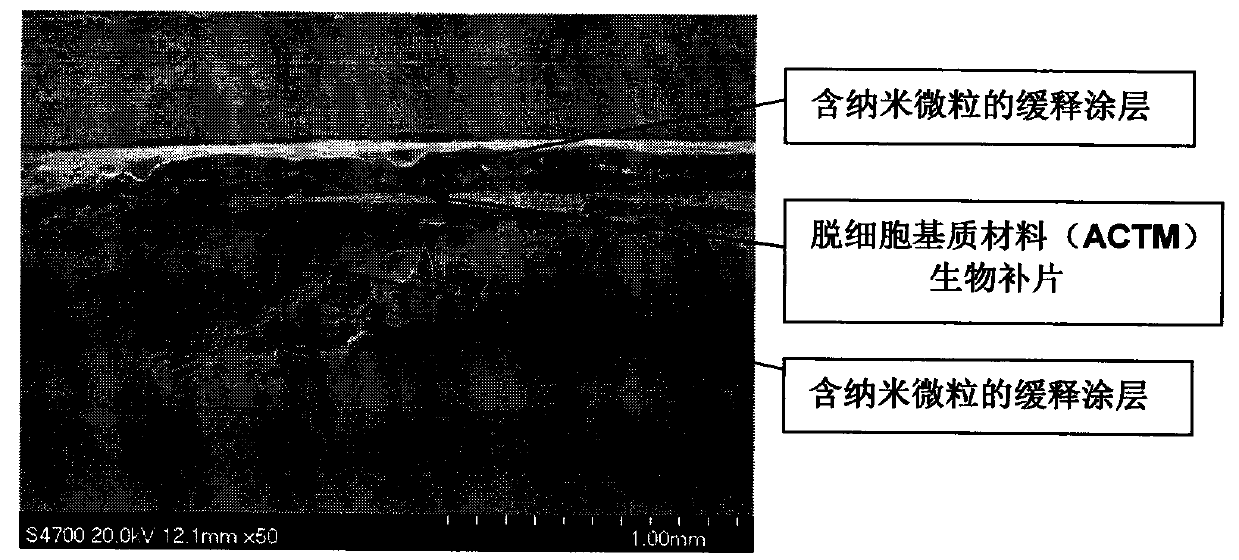
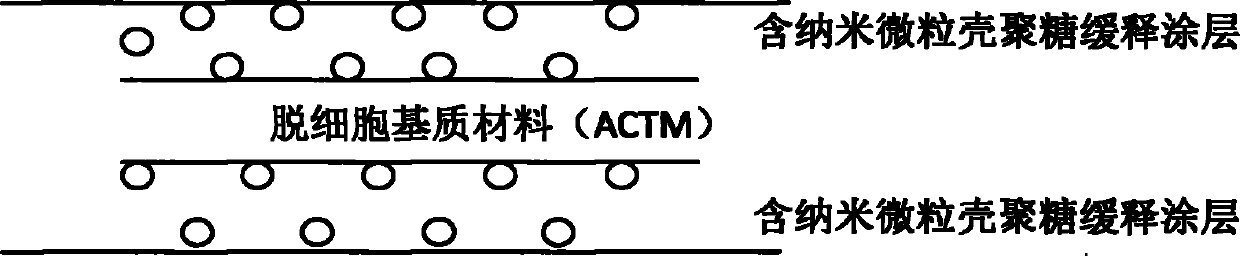
Anti-tumor biological patch
A technology of biological patches and anti-tumor drugs, which is applied in the field of anti-tumor biological patches, can solve the problems of non-feasibility, increased recurrence of tumor cell implantation, etc., and achieve the effect of less side effects and increased curative effect of chemotherapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of anti-tumor biological patch
[0033] A. Methods of coating and dip-dyeing patches
[0034] A typical method of compositing a patch using a square 8cmx10cm is described below. For larger patches, a larger volume of polymer / drug solution is used.
[0035] Briefly, sufficient polymer was weighed directly into a 20ml glass scintillation vial and enough DCM was added to obtain a 2% w / v solution. The vial was then capped and mixed by hand to dissolve the polymer. The patch was then held in a vertical orientation using micro-clamps attached to a fixture 6-12 inches above the fume hood platform to enable horizontal spraying. Using an automatic pipette, transfer an appropriate volume (minimum 5ml) of the 2% polymer solution into a separate 20ml glass scintillation vial. An appropriate amount of nanoparticles of 5-fluorouracil combined with rapamycin was then added to the solution and mixed by hand shaking.
[0036] To prepare for nebulization, the cap of the ...
Embodiment 2
[0041] In vitro drug release rate
[0042] Small pieces (0.5x0.5 cm) of patches (n=4) coated with nanoparticles of 5-fluorouracil combined with rapamycin were placed in 14 ml glass tubes, followed by the addition of 10 ml phosphate containing 0.4 g / L albumin Buffered saline (PBS, pH=7.4). Tubes were incubated at 37°C with gentle swirl mixing at 8 rpm. At regular intervals, 10 ml of the supernatant was removed for analysis of 5-fluorouracil and rapamycin and replaced with fresh PBS / albumin buffer. 1 ml of dichloromethane was added to the removed supernatant and the tube was capped and shaken by hand for 1 minute to allow all released 5-fluorouracil and rapamycin to partition into the separate dichloromethane phase. The tubes were then centrifuged at 500 x g for 1 min, 10 ml of the upper aqueous phase was removed and discarded, and the dichloromethane phase was evaporated at 50 °C for 20 min under nitrogen. 1 ml of 60% acetonitrile in water (v / v) was added to each tube to dis...
Embodiment 3
[0044] In vivo supplement effect
[0045] General anesthesia was introduced into domestic pigs. Shave the abdominal area and disinfect the skin with a wash. Under aseptic conditions, a vertical incision was made on one side of the abdomen to expose the abdominal cavity. The synthetic mesh is repaired in the abdominal wall or replaces the peritoneum to contact the viscera of the abdominal cavity. Animals were randomly divided into 4 groups of 5 pigs each receiving synthetic patches coated with (1) carrier polymer only; Carrier polymer; (3) loading 5% 5-fluorouracil and rapamycin nanoparticle carrier polymer or (4) loading 10% 5-fluorouracil and rapamycin nanoparticle carrier polymer. Shut up.
[0046] A contralateral abdominal incision was made in the same manner and a control uncoated patch was used to repair the abdominal wall or replace the peritoneum. Let the animal recover.
[0047] Animals were sacrificed at 1 month, 3 months and 6 months after operation. Remove th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap