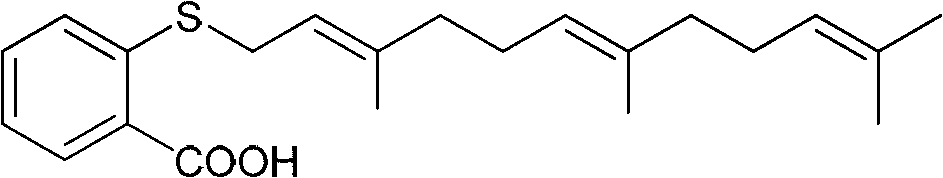
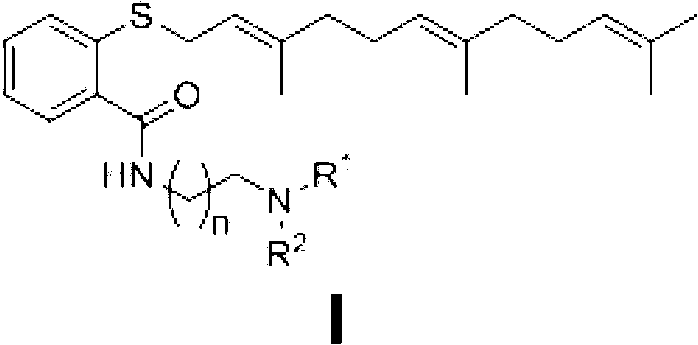
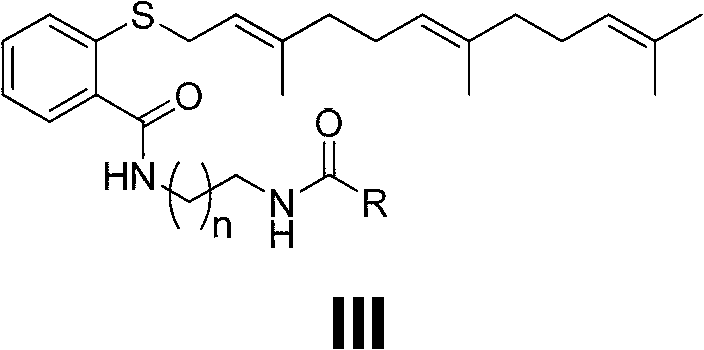
Novel diamine-containing farnesyl thiosalicylic acid derivative and preparation method and medicinal application thereof
A kind of technology of derivatives and diamines, applied in the application field of preparing antitumor drugs and neuroprotective drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0138] The preparation of embodiment 1 farnesyl thiosalicylic acid chloride
[0139] Dissolve 0.54 g (1.50 mmol) FTA in 10 mL anhydrous CH 2 Cl 2 0.60 mL (8.27 mmol) of thionyl chloride was added thereto, stirred at 55° C. for 1 hour, and concentrated to obtain farnesylthiosalicylic acid chloride as a yellow oil.
Embodiment 2
[0140] The preparation of embodiment 2N-(2-aminoethyl) farnesyl thiosalicylic acid amide (I1)
[0141] Dissolve 0.30 g (5 mmol) of 1,2-ethylenediamine and 0.20 mL (1.50 mmol) of triethylamine in 5 mL of anhydrous CH 2 Cl 2 0.38 g (1.0 mmol) of farnesyl thiosalicylic acid chloride was slowly added dropwise to 10 mL of anhydrous CH 2 Cl 2 solution, then stirred at room temperature for 1.5 h, concentrated the solvent, evaporated the remaining ethylenediamine, and obtained 0.28 g of yellow oil by column chromatography, with a yield of 70.5%.
[0142] 1 H NMR (CDCl 3 ,300MHz):8.00(m,1H,Ar-H),7.78(m,1H,Ar-H),7.38(m,2H,Ar-H),5.23(m,1H,SCH 2 C H ), 5.08(m, 2H, 2×CH 2 C H =CCH 3 ), 3.56 (d, 2H, J=9.0Hz, SCH 2 ), 2.99-3.05 (m, 4H, 2×NCH 2 ),1.99(m,8H,2×CHC H 2 C H 2 CH),1.53-1.68(m,12H,4×CH 3 );ESI-MS(m / z):401[M+H] + .
Embodiment 3
[0143] Embodiment 3N-(3-aminopropyl) farnesyl thiosalicylic acid amide (I 2 ) preparation
[0144] Referring to Example 2N-(2-aminoethyl) farnesyl thiosalicylic acid amide (I 1 ) preparation method, by reacting 1,2-ethylenediamine and farnesyl thiosalicylic acid chloride in the 1,3-propanediamine substitution method to prepare light yellow oil N-(3-aminopropyl) Farnesylthiosalicylic acid amide (I 2 ), the yield was 70.1%.
[0145] 1 H NMR (CDCl 3 ,300MHz):7.93(m,2H,Ar-H),7.32(m,2H,Ar-H),5.21(m,1H,SCH 2 C H ),5.12(m,2H,2×CH 2 C H =CCH 3 ), 3.51 (d, 2H, J=9.0Hz, SCH 2 ),3.05(m,4H,2×NC H 2 ),1.90-1.99(m,8H,2×CHC H 2 C H 2 CH),1.54-1.69(m,14H,4×CH 3 ,CH 2 C H 2 CH 2 );ESI-MS(m / z):415[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com