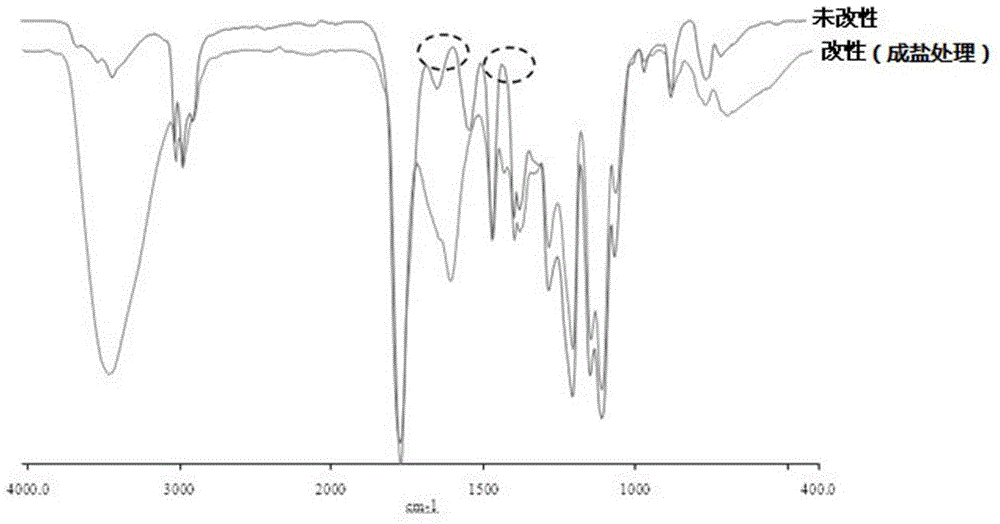
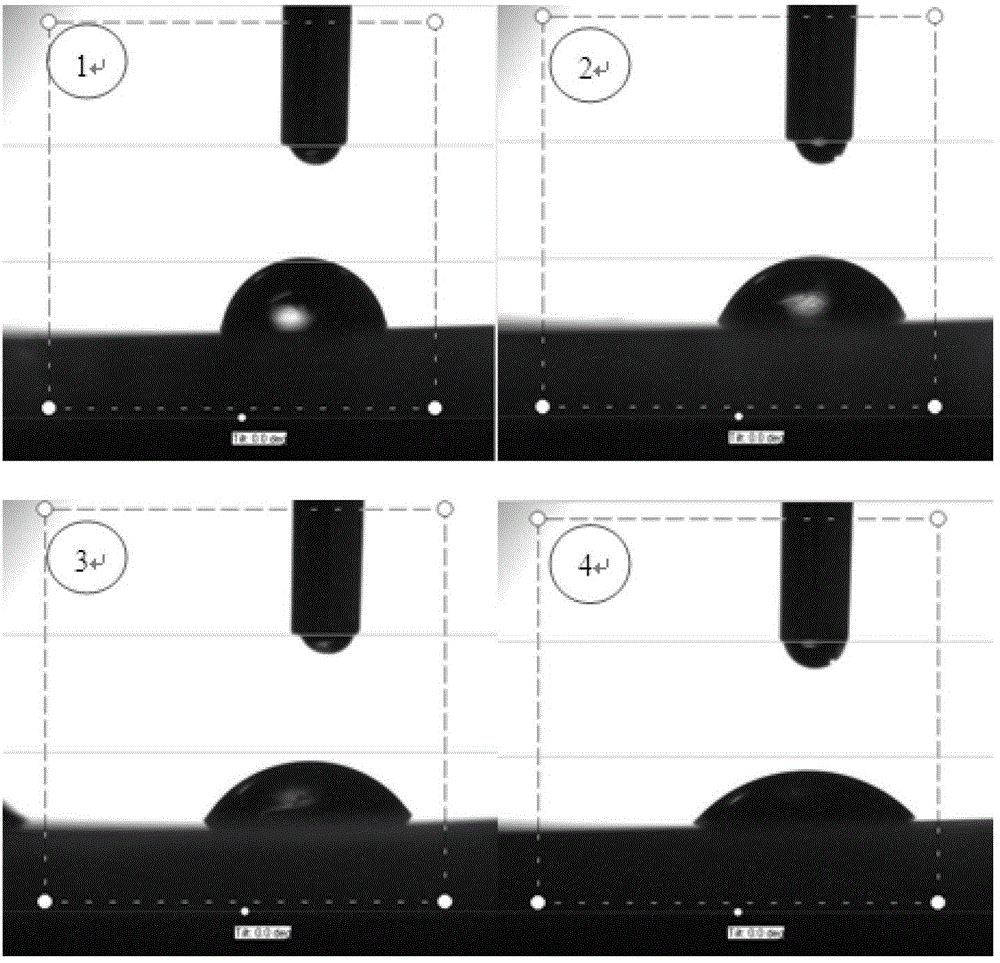
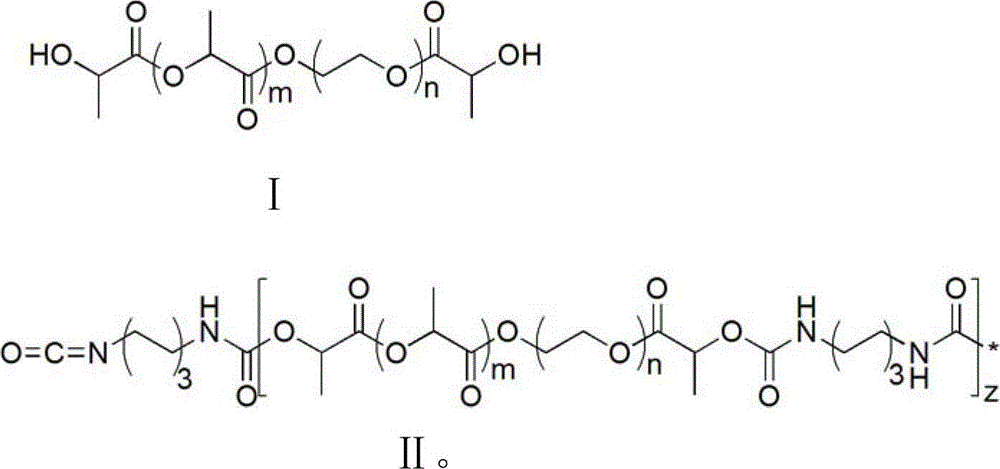
2, 2-dihydromethyl propionic acid modified shape memory polyurethane urea material and method for preparing same
A technology of dimethylol propionic acid and polyurethane urea, which is applied in the field of chemistry, can solve the problems of affecting the mechanical properties of materials, disappearing of osteoinductive effect, and is not a solution, so as to achieve excellent hydrolysis resistance, improve biological activity, and improve mechanical properties. The effect of intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Accurately weigh D, L-lactide 14.400g (molecular weight 144, distilled and ethyl acetate recrystallization 3 times before weighing) polyethylene glycol 2.667g (molecular weight 400, weighed) according to the molar ratio of 15:1. Molecular sieve to remove water), respectively added to 100ml round bottom flask, add initiator stannous octoate according to 1 / 5000 of the molar weight of D, L-lactide, put it into an oil bath at 130°C for reaction after vacuum sealing 24h. After the reaction, the flask was taken out, cooled naturally and dissolved in dichloromethane, extracted in absolute ethanol, and vacuum-dried to obtain 13.610 g of hydroxyl-terminated macromolecular alcohol. Its molecular weight is 2450 through NMR analysis.
Embodiment 2
[0041] Accurately weigh D, L-lactide 14.400g (molecular weight 144, distilled and ethyl acetate recrystallization 3 times before weighing) polyethylene glycol 2.000g (molecular weight 400, weighed) according to the molar ratio of 20:1. Molecular sieve to remove water), respectively added to 100ml round bottom flask, add initiator stannous octoate according to 1 / 5000 of the molar weight of D, L-lactide, put it into an oil bath at 130°C for reaction after vacuum sealing 24h. After the reaction, the flask was taken out, cooled naturally, dissolved in dichloromethane, extracted in absolute ethanol, and dried in vacuum to obtain 14.013 g of hydroxyl-terminated macromolecular alcohol. Its molecular weight is 2956 according to NMR analysis.
Embodiment 3
[0043]Accurately weigh D, L-lactide 14.400g (molecular weight 144, distilled and ethyl acetate recrystallization 3 times before weighing) polyethylene glycol 1.333g (molecular weight 400, weighed) according to the molar ratio of 30:1. Molecular sieve to remove water), respectively added to 100ml round bottom flask, add initiator stannous octoate according to 1 / 5000 of the molar weight of D, L-lactide, put it into an oil bath at 130°C for reaction after vacuum sealing 24h. After the reaction, the flask was taken out, cooled naturally, and dichloromethane was added to dissolve, extracted in absolute ethanol, and vacuum-dried to obtain 14.408 g of hydroxyl-terminated macromolecule alcohol. Its molecular weight was 4241 by NMR analysis.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Static water contact angle | aaaaa | aaaaa |
| Static water contact angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap