4-aminoquinazoline compound and preparation method and application thereof
A technology of aminoquinazolines and compounds, which is applied in the field of drug synthesis, and can solve the problems that anti-tumor drugs cannot meet the treatment requirements.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
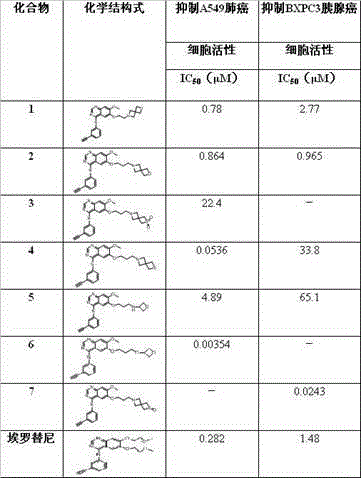
[0038] Example 1: Synthesis of Compounds 1 , (3-ethynyl-phenyl)-{7-methoxy-6-[3-(2-oxa-6-aza-spiro[3.3]heptane-6-yl)-ethoxy] - Quinazolin-4-yl}-amine
[0039] 1) Synthesis of 6,7-dimethoxy-3H-quinazolin-4-one
[0040] Methyl 2-amino-4,5-dimethoxybenzoate (31.5g, 149.1mmol) and amine formate (9.45 g, 149.0 mmol) in formamide (105ml) were reacted by heating at 200 °C for 2 hours. Then cool to room temperature, filter the reaction solution, wash the filter cake with water, and dry to obtain the product (26.3 g, 85.6%).
[0041] 2) Synthesis of 6-hydroxy-7-methoxy-3H-quinazolin-4-one.
[0042] Add 6,7-dimethoxy-3H-quinazolin-4-one (26.3 g, 127.5 mmol) and L-methionine (22.8 g, 153.0 mmol) to a solution of methanesulfonic acid (175.0 mL) In the reaction bottle, then heated and stirred at 100 ° C for 22 hours to the end of the reaction. After cooling the reaction solution to room temperature, the reaction solution was poured into a container filled with 1L ice, and continued to...
Embodiment 2
[0058] Embodiment 2: synthetic compound 2 , (3-ethynyl-phenyl)-{7-methoxy-6-[2-(2-oxa-6-aza-spiro[3.3]heptane-6-yl)-propoxy] - Quinazolin-4-yl}-amine
[0059] Add [6-(3-bromo-propoxy)-7-methoxy-quinazolin-4-yl]-(3-ethynyl-phenyl)-amine (50 mg), acetonitrile (10ml), Na 2 CO 3 (250 mg) and 2-oxa-6-aza-spiro[3.3]heptane oxalate (50 mg). The reaction was heated at 70°C for 1 hour and the product (34 mg) was obtained by preparative HPLC. LC-MS (m / z): 431(M+). 1 H NMR (d6-DMSO, 400 MHz): 1.81(t, J=6.4Hz, 2H), 2.49(m,2H), 3.28(s,4H), 3.94(s,3H), 4.15(t, J= 6.4Hz, 2H), 4.21(s,1H), 4.60(s,4H), 7.21(m,2H), 7.41(t, J=8Hz, 1H), 7.82-7.99(m,3H) , 8.50(s ,1H), 9.53(s,1H). 13 C NMR (CD 3 OD, 400 MHz): 26.71, 38.859, 55.126, 55.401, 62.938, 66.690, 77.360, 80.853, 82.992, 101.897, 105.757, 109.099, 122.774, 125.539, 127.237, 128.471, 139.282, 146.202, 148.962, 152.420, 155.321, 156.928 。
[0060]
Embodiment 3
[0061] Embodiment 3: synthetic compound 3 , {6-[3-(2-sulfone hetero-6-aza-spiro[3.3]heptane-6-yl)-propoxy]-7-methoxy-quinazolin-4-yl}- (3-Ethynyl-phenyl)-amine
[0062] Add [6-(3-bromo-propoxy)-7-methoxy-quinazolin-4-yl]-(3-ethynyl-phenyl)-amine (50 mg), acetonitrile (10ml), Na 2 CO 3 (250 mg) and 2-sulfone-6-aza-spiro[3.3]heptane oxalate (50 mg). The reaction was heated at 70°C for 1 hour and the product (6 mg) was obtained by preparative HPLC. LC-MS (m / z): 479(M+). 1 H NMR (CD 3 OD, 400 MHz): 1.98(m, 2H), 2.75-2.79(m,2H), 3.52(m,4H), 4.01(s,3H), 4.23(m,2H), 4.30(m,4H), 7.19(s,1H), 7.21-7.29(m,1H), 7.40(t, J=8Hz, 1H), 7.74-7.80(m,2H), 7.93(m,1H), 8.45(s,1H). 13 C NMR (CD 3 OD, 400 MHz): 24.818, 26.760, 55.169, 64.065, 66.722, 72.952, 77.304, 102.015, 105.799, 109.179, 122.859, 125.684, 127.355, 128.513, 139.243, 146.322, 149.098, 152.490, 155.480, 157.116。
[0063]
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap