Method for increasing activity in human stem cell
A high-activity, high-quality stem cell technology, applied in the field of preparation of high-activity human mesenchymal stem cell clumps, can solve the problems of not being able to meet the requirements of use, changes, cell aging, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1 Inducing sphere formation of human mesenchymal stem cells
[0071] (1) Medium for inducing spheroid formation
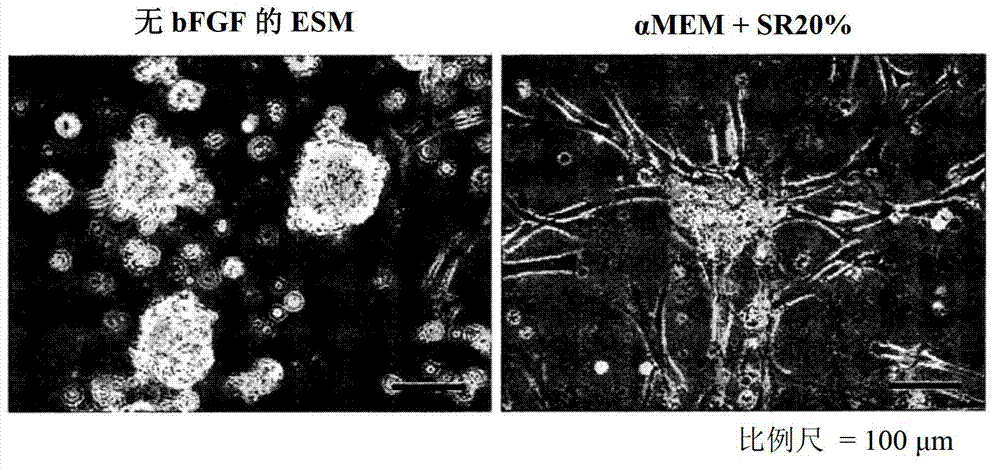
[0072] Using low-adsorption culture dishes for non-adherent culture, the above-mentioned mesenchymal stem cells were cultured in the traditional medium of mesenchymal stem cells, that is, α-MEM medium (Invitrogen) supplemented with serum substitute (SR). However, induction of non-adherence was unsuccessful (see figure 1 A).
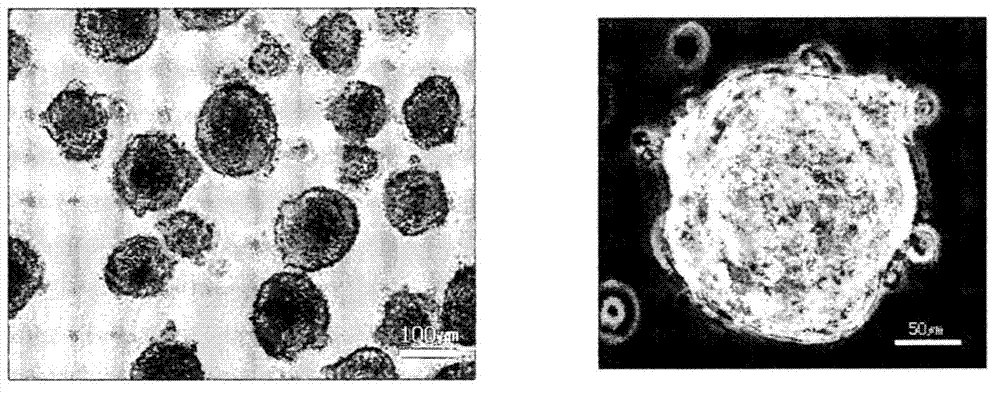
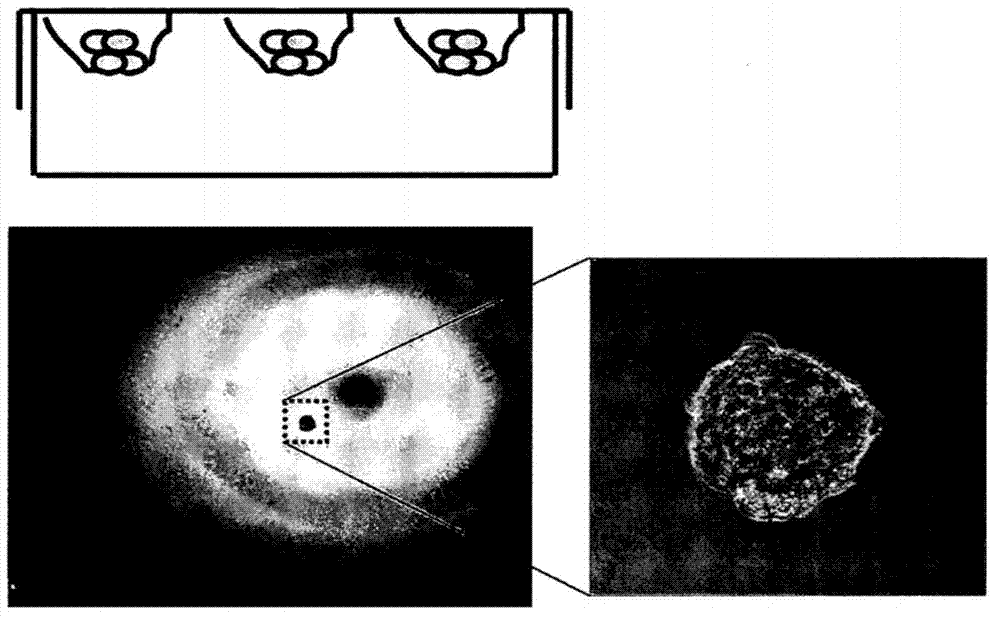
[0073] Next, use low-adhesion culture dishes for non-adherent culture, and culture mesenchymal stem cells in embryonic stem cell medium (ESM) with basic fibroblast growth factor (bFGF) removed (hereinafter referred to as bFGF-free ESM) . Successfully induced non-adherence (see figure 1 B). The medium did not contain any fetal bovine serum (FBS), but contained DMEM / F-12 (Invitrogen), 20% KnockOut SR (Invitrogen), 0.1 mmol / L β-mercaptoethanol (Sigma), 1% non-essential amino acids (Invitrogen ), 50 IU / mL penicillin and 50 m...
Embodiment 2
[0078] Example 2 Effect of spheroid formation - in vivo activity
[0079] Evaluation of in vivo activity of mesenchymal stem cells in a rat model of ischemic heart disease. Rat models of ischemic heart disease were prepared by inducing ischemia by coronary artery ligation.
[0080] The rat model of ischemic heart disease was divided into three groups for evaluation: one group was injected with spherical cell aggregates (spheroids) prepared in (2) in Example 1; one group was injected with single cells prepared from spherical cell aggregates ( Dissociate) (Dissociate); a group of cells whose injection did not induce the formation of any spherical cell clumps (blank control group) ( ). At least 7 rats per group.
[0081] (1) ECG measurement
[0082] The baseline electrocardiogram was measured 4 days after the rat model was prepared, and the rats were injected with stem cells 7 days after the rat model was prepared. Specifically, stem cells or cell clumps were injected int...
Embodiment 3
[0101] Example 3: Analysis of spheroid formation mechanism (in vivo activity)
[0102] Experiments were conducted to analyze the mechanism of spheroid formation, which can lead to different in vivo activities as shown in Example 2.
[0103] First, EDTA was added to stem cells that sequestered calcium ions, which play an important role as a cell adhesion factor, by inducing spheroid formation in bFGF-free ESM using a low-adsorption culture dish. Such as Figure 11 As shown, in the presence of EDTA, no spheroids were formed. In other words, since as Ca 2+ The addition of chelating agent EDTA, the spheroid formation of mesenchymal stem cells was blocked, thus it is considered that spheroid formation is through Ca 2+ dependent on cell adhesion molecules.
[0104] From this, detection of two distinct Ca in spheroid formation by Western blot analysis 2+ Expression of dependent cell adhesion molecules, namely neural cadherin and epithelial cadherin. Such as Figure 12 showed t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com