O-carboxymethyl chitosan-stearic acid polymer as well as synthesis method thereof and application of polymer
A technology of carboxymethyl chitosan and stearic acid is applied in the synthesis field of nanometer polymer micelle materials (O-carboxymethyl chitosan-stearic acid polymer), and can solve the problems such as restricting popularization and application. , to achieve the effect of simple and convenient preparation process, uniform distribution and small particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The synthesis of embodiment 1 O-carboxymethyl chitosan-stearic acid polymer
[0033] (1) Weigh 250mg of O-carboxymethyl chitosan and dissolve it in 30mL of distilled water, stir to fully swell and dissolve, then heat to 65°C in a water bath;
[0034] (2) Dissolve 248mg of stearic acid in 20mL of absolute ethanol, then add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride equivalent to 1.5 times the molar amount of stearic acid Salt and N-hydroxysuccinimide equivalent to 1.5 times the molar amount of stearic acid, heated to 65°C to dissolve completely;
[0035] (3) Add the transparent solution obtained in the above step (2) dropwise into the aqueous solution of O-carboxymethyl chitosan in step (1) under strong stirring, react at 65°C for 5 hours, dialyze with distilled water for 3 days, and then freeze-drying, and washing the dried product with absolute ethanol to obtain O-carboxymethyl chitosan-stearic acid polymer.
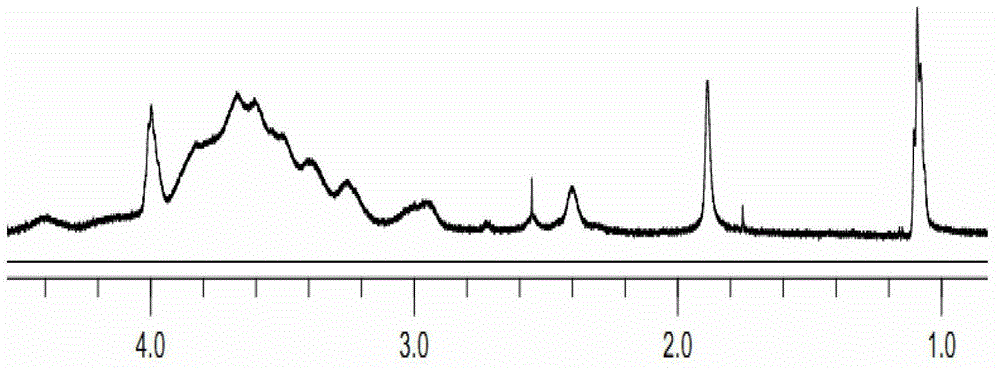
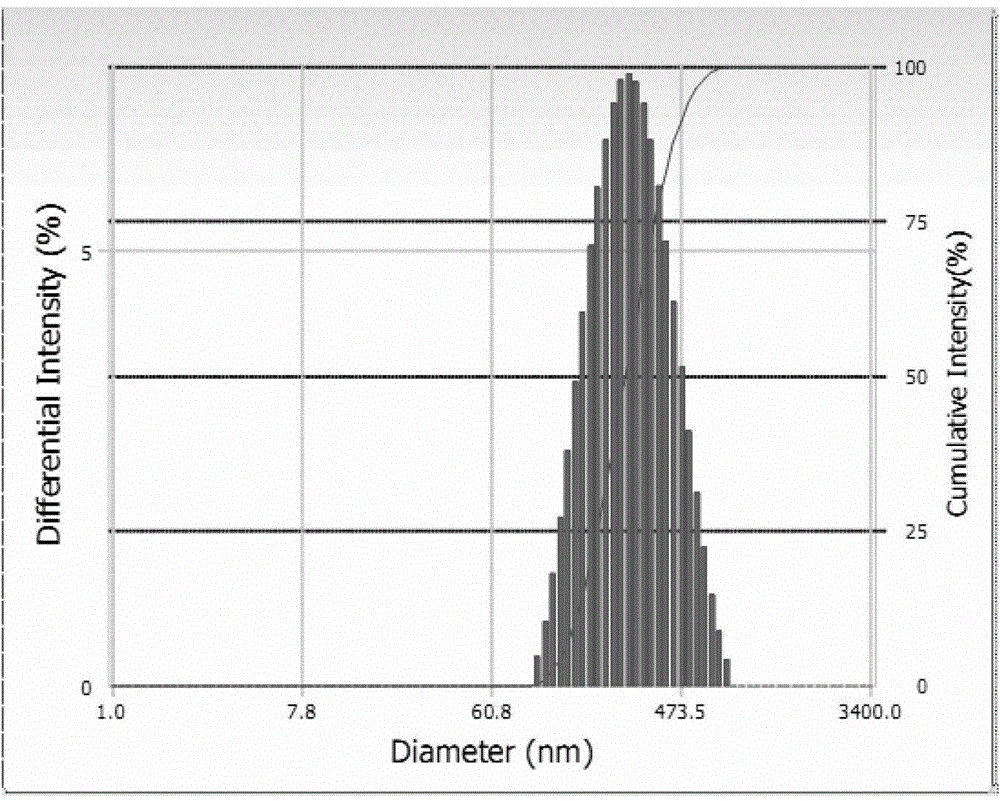
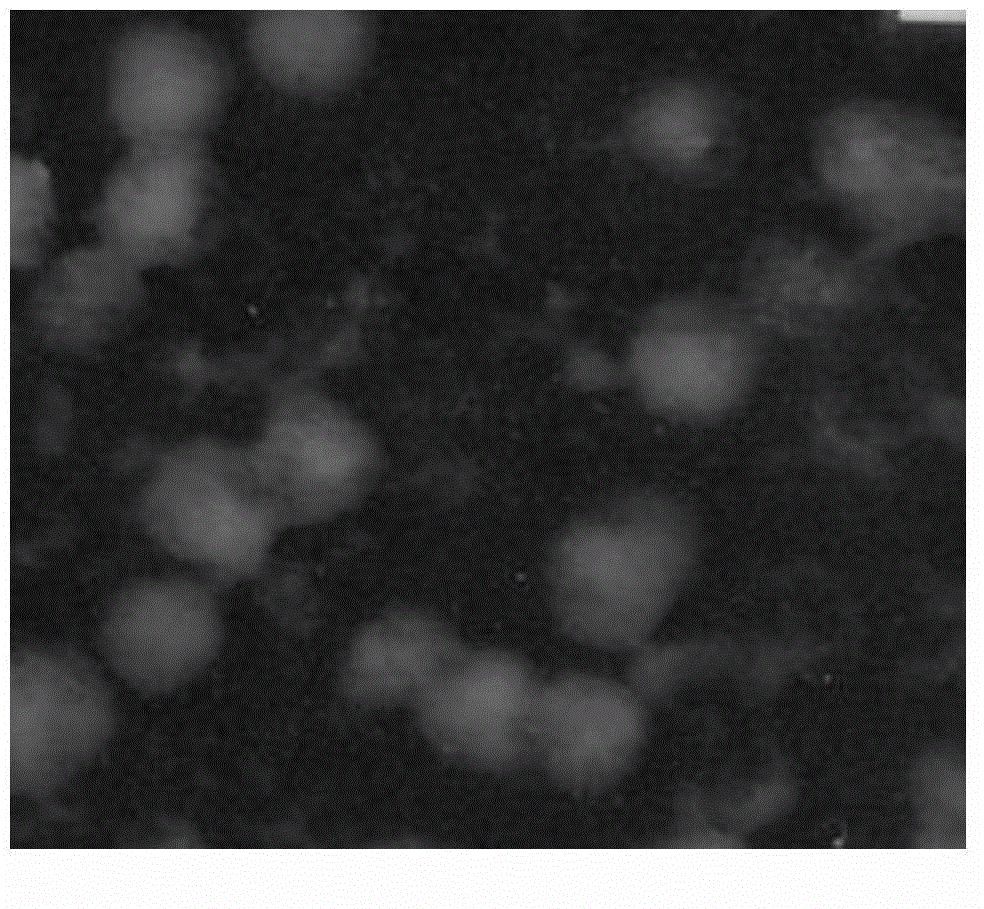
[0036] Gained O-carboxymethyl chitosan-s...
Embodiment 2
[0037] The synthesis of embodiment 2 O-carboxymethyl chitosan-stearic acid polymer
[0038] (1) Weigh 250mg of O-carboxymethyl chitosan and dissolve it in 30mL of distilled water, stir to fully swell and dissolve, then heat to 65°C in a water bath;
[0039](2) Dissolve 330mg of stearic acid in 20mL of absolute ethanol, then add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride equivalent to 1.5 times the molar amount of stearic acid Salt and N-hydroxysuccinimide equivalent to 1.5 times the molar amount of stearic acid, heated to 65°C to dissolve completely;
[0040] (3) Add the transparent solution obtained in the above step (2) dropwise into the aqueous solution of O-carboxymethyl chitosan in step (1) under strong stirring, react at 65°C for 5 hours, dialyze with distilled water for 3 days, and then freeze-drying, and washing the dried product with absolute ethanol to obtain O-carboxymethyl chitosan-stearic acid polymer.
Embodiment 3
[0041] The synthesis of embodiment 3 O-carboxymethyl chitosan-stearic acid polymer
[0042] (1) Weigh 250mg of O-carboxymethyl chitosan and dissolve it in 30mL of distilled water, stir to fully swell and dissolve, then heat to 65°C in a water bath;
[0043] (2) Dissolve 130mg of stearic acid in 20mL of absolute ethanol, then add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride equivalent to 1.5 times the molar amount of stearic acid Salt and N-hydroxysuccinimide equivalent to 1.5 times the molar amount of stearic acid, heated to 65°C to dissolve completely;
[0044] (3) Add the transparent solution obtained in the above step (2) dropwise into the aqueous solution of O-carboxymethyl chitosan in step (1) under strong stirring, react at 65°C for 5 hours, dialyze with distilled water for 3 days, and then freeze-drying, and washing the dried product with absolute ethanol to obtain O-carboxymethyl chitosan-stearic acid polymer.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| degree of carboxylation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com