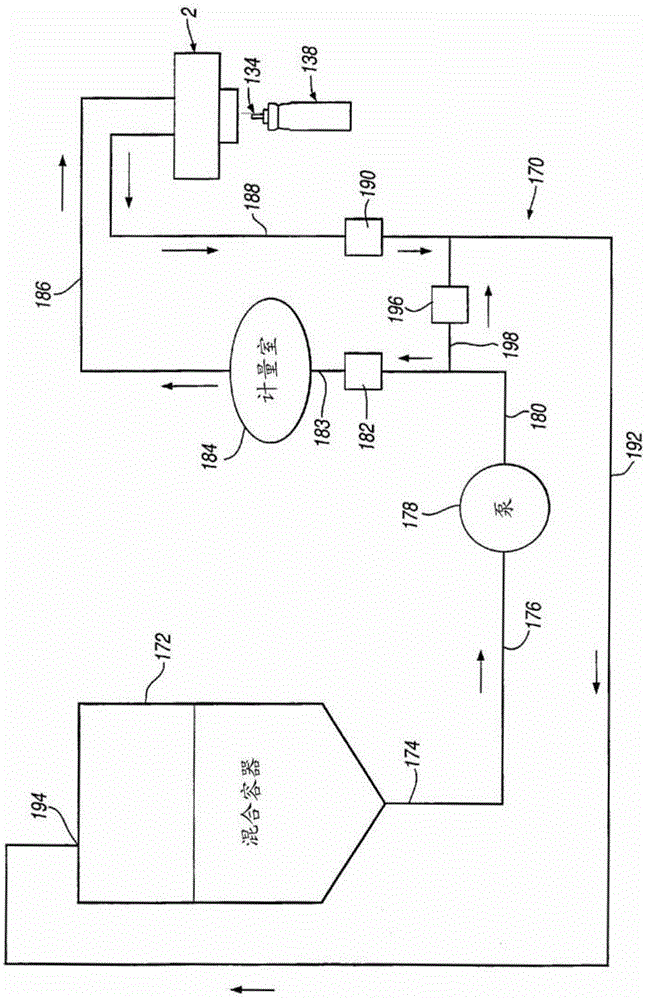
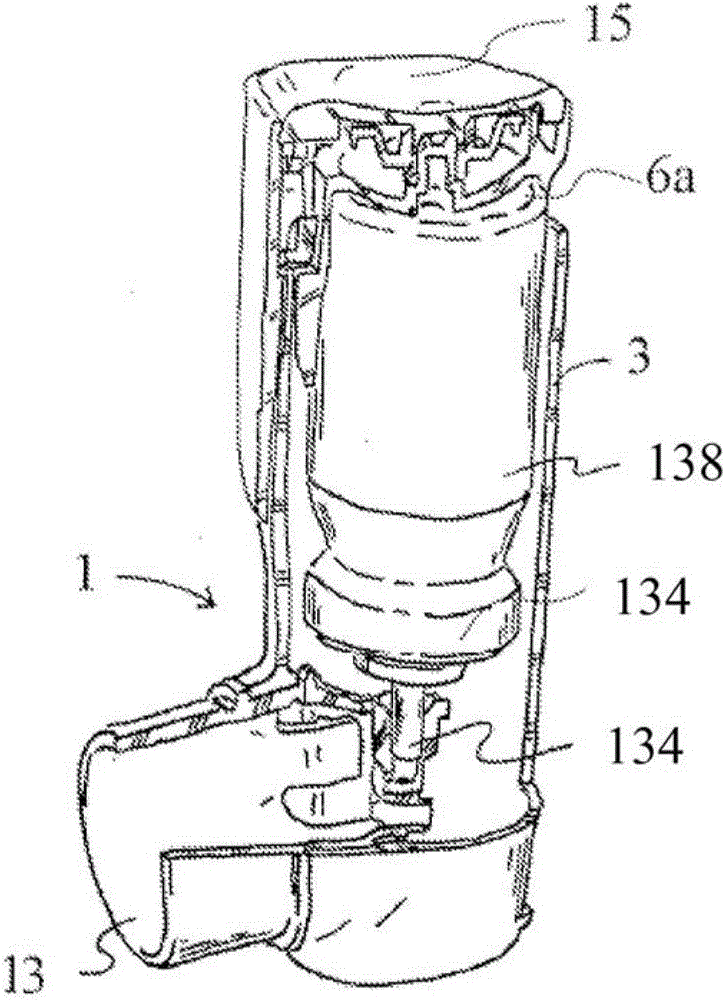
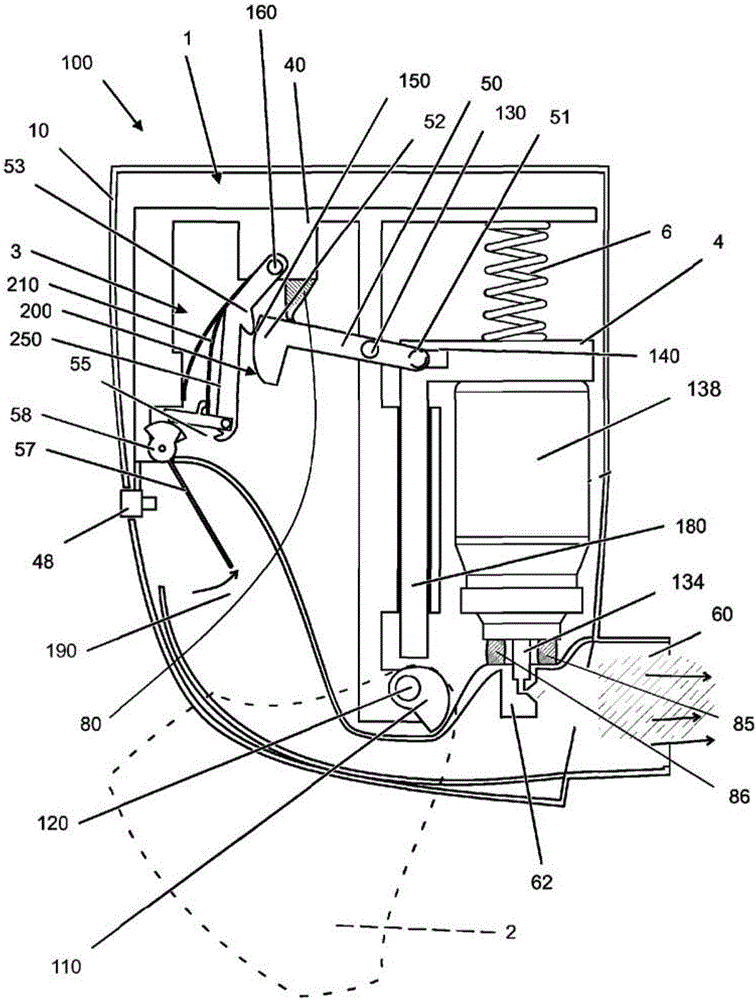
Provides a method of filling a canister for an inhaler
A technology for filling canisters and inhalers, applied in the field of pressurized metered dose inhalers, providing canisters and filling the canisters with pharmaceutical components, which can solve the problems of time-consuming, expensive and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] The three canisters for the three inhalers were assembled and filled with drug in a conventional manner, ie using a decontamination step in which the canisters were purged with propellant, after which the canisters were sealed with metering valves and filled with drug. Test each inhaler as follows:
[0091] 1. Actuate in the conventional manner to dispense an aerosol.
[0092] 2. Actuate again and hold in the actuated position (ie, where the metering valve remains open) for a first predetermined period of time (recorded in column 1 of Table 1).
[0093] 3. Weighing.
[0094] 4. Actuate again and hold in the actuated position (ie, where the metering valve remains open) for a first predetermined period of time (recorded in column 1 of Table 1).
[0095] 5. Weighing.
[0096] 6. Drive in the usual way.
[0097] 7. Weighing.
[0098] Drive weight over hold time was calculated as follows: inhaler weight of step 5 minus inhaler weight of step 3 and inhaler weight of step...
Embodiment 2
[0104] For comparison with Example 1, a canister for an inhaler was assembled and filled with propellant according to aspects of the invention. The canister was configured and filled by the same method as Example 1, except that there was no decontamination step, whereby the canister of the inhaler in Example 2 was not decontaminated. As described in Example 1, the inhaler was tested in Example 2 in the following manner:
[0105] 1. Actuate in the conventional manner to dispense an aerosol.
[0106] 2. Re-actuate and hold in the actuated position (ie, where the metering valve remains open) for a first predetermined period of time (recorded in column 1 of Table 2).
[0107] 3. Weighing.
[0108] 4. Actuate again and hold in the actuated position (ie, where the metering valve remains open) for a first predetermined period of time (recorded in column 1 of Table 2).
[0109] 5. Weighing.
[0110] 6. Drive in the usual way.
[0111] 7. Weighing.
[0112] Drive weight over hold...
Embodiment 3
[0118] As discussed above, the aerosol actuation weight has a direct effect on the active pharmaceutical ingredient in the delivered dose of drug, and thus the patient receives a potential drug dose with each actuation. To confirm this, 90 purified tanks and 30 unpurified tanks (for comparison) were configured in the same manner as above. Each canister contains the same predetermined and known number of doses of the selected drug, thus allowing the beginning, middle and end of the canister's useful life to be determined.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com