Qianxi capsule and preparation method thereof
A technology of Qianxi capsules and capsules, applied in the field of medicine, can solve the problems of complicated auxiliary material process, affecting the coating process, poor friability of plain tablets, etc., and achieve the effects of large evaporation surface area, short heating time and low hygroscopicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1 A kind of preparation method of Qianxi Capsule
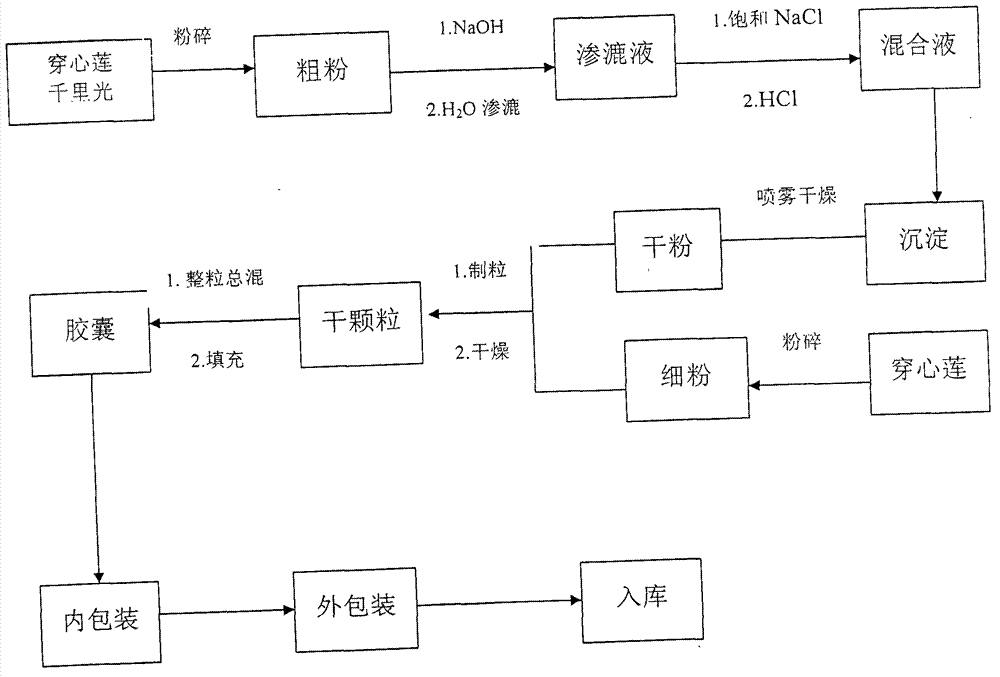
[0027] Take Andrographis paniculata 40g and pulverize into fine powder, sieve; 1960 grams of Andrographis paniculata and 2000 grams of Senecio are pulverized into coarse powder, soak with 1.2% sodium hydroxide solution in right amount overnight, add water to percolate, flow rate is 3ml / min per kilogram of medicinal material, collect Percolate about 1000ml, add 1 / 7 amount of saturated saline, then adjust the pH to about 2-3 with hydrochloric acid, stir well, let it stand for 12 hours, discard the supernatant, collect the precipitate, spray dry; add the Andrographis paniculata Fine powder, 30 grams of starch, 7.5 grams of gelatin, mixed evenly, made into granules, dried, filled with 1000 capsules, packed in aluminum-plastic.
Embodiment 2
[0028] Embodiment 2 A kind of preparation method of Qianxi Capsule
[0029] Take 40g of Andrographis paniculata and crush it into fine powder, sieve; the remaining 1960g of Andrographis paniculata and 2000g of Senecio are crushed into coarse powder, soak overnight with an appropriate amount of 1.3% sodium hydroxide solution, add water to percolate at a flow rate of 5ml / min per kilogram of medicinal material, collect Percolate about 1000ml, add 1 / 7 amount of saturated saline, then adjust the pH to about 2-3 with hydrochloric acid, stir well, let it stand for 12 hours, discard the supernatant, collect the precipitate, spray dry; add the Andrographis paniculata Fine powder, 28 grams of starch, 7 grams of gelatin, mixed evenly, made into granules, dried, filled with 1000 granules, and packed in aluminum-plastic.
Embodiment 3
[0031] 3.1 Materials
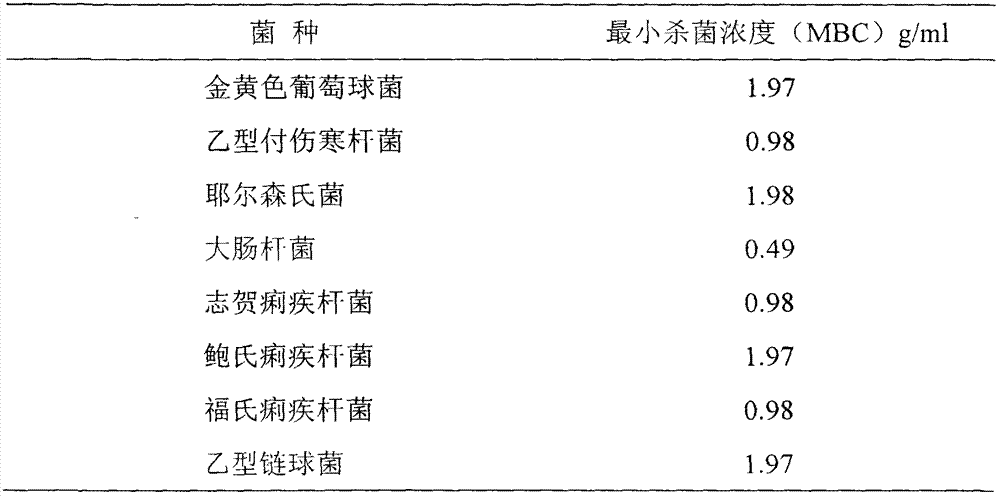
[0032] Bacteria: Staphylococcus aureus (26003), Escherichia coli (44103), typhoid bacillus (0602-10), Yersinia (52301-5), Shigella dysenteriae (1507-2), Shigella baumannii (51583), Shigella flexneri (51571) (the above strains were all obtained from the China Institute for the Control of Pharmaceutical and Biological Products, Ministry of Health), and Streptococcus B (from the Baozhi strains of the Department of Microbiology, Jiangxi University of Traditional Chinese Medicine).
[0033] Test substance: Qianxi Capsule
[0034] 3.2 Method
[0035] Suspend Qianxi Capsules in 100ml broth, dilute the drug according to the double dilution method, and use the other tube without drug as a control, and dilute the cultures of 9 bacterial strains cultivated for 6 hours to 10 -4 , each tube was inoculated with 0.1ml, incubated for 24 hours, then inoculated in a flat container, incubated at 37°C for 24 hours, determined whether there were colonies, and measured the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com