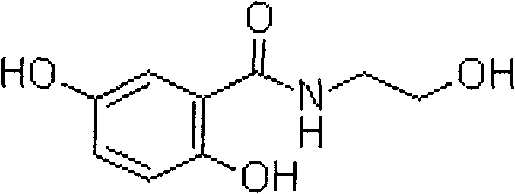
2,5-dihydroxy-n-(2-hydroxyethyl)benzamide preparation method
A technology of gentisic acid ethanolamine and ethanolamine is applied in the preparation of carboxylic acid amides, the preparation of organic compounds, chemical instruments and methods, etc., and can solve the problems of difficult purification of reaction products, difficult operation of synthesis process, difficulty in separation and purification of final products, and the like, To achieve the effect of reducing cost and environmental pollution, easy availability of raw materials and improving quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 200g of methyl gentisate into a 500ml single-necked bottle, drop in 79.9g of ethanolamine, heat slightly, wait until the solids are completely dissolved, raise the temperature to 80°C, keep stirring for 8 hours, monitor by TLC, until the reaction is complete, dissolve with 200ml of purified water, overnight Crystallize, filter with suction, wash with water twice, and dry under vacuum at 50°C for 4 hours to obtain 160.6 g of the product with a yield of 68.44%.
[0023] Spectral data for structural confirmation of the product: 1 H NMR (400MHz, DMSO-d 6 )δ: 3.33 ~ 3.36 (m, 2H, -CH 2 -), 3.49~3.53(m, 2H, -CH 2 -), 4.74~4.76(m, 1H, -OH), 6.71~6.73(d, 1H, =CH-), 6.82~6.84(d, 1H, =CH-), 7.25(s, 1H, =CH- ), 8.67(s, 1H, 1H, -OH), 8.95(s, 1H, -OH), 11.57(s, 1H, -NH-), the results are consistent with the structure.
Embodiment 2
[0025] Add 200g of methyl gentisate into a 1000ml single-necked bottle, and then add 200ml of water; raise the temperature to 80°C, add 79.9g of ethanolamine dropwise, keep stirring for 8 hours, monitor by TLC, until the reaction is complete, dissolve in 200ml of purified water, and crystallize overnight. Suction filtration, washing with water twice, and vacuum drying at 50° C. for 4 hours gave 170.0 g of the product with a yield of 72.44%. The structure of the product was confirmed to be consistent.
Embodiment 3
[0027] Add 200g of ethyl gentisate into a 1000ml single-necked bottle, and then add 200ml of water; raise the temperature to 80°C, add 79.9g of ethanolamine dropwise, keep stirring for 8 hours, monitor by TLC, until the reaction is complete, dissolve in 200ml of purified water, and crystallize overnight. Suction filtration, washing with water twice, and vacuum drying at 50° C. for 4 hours gave 150.0 g of the product with a yield of 63.92%. The structure of the product was confirmed to be consistent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com