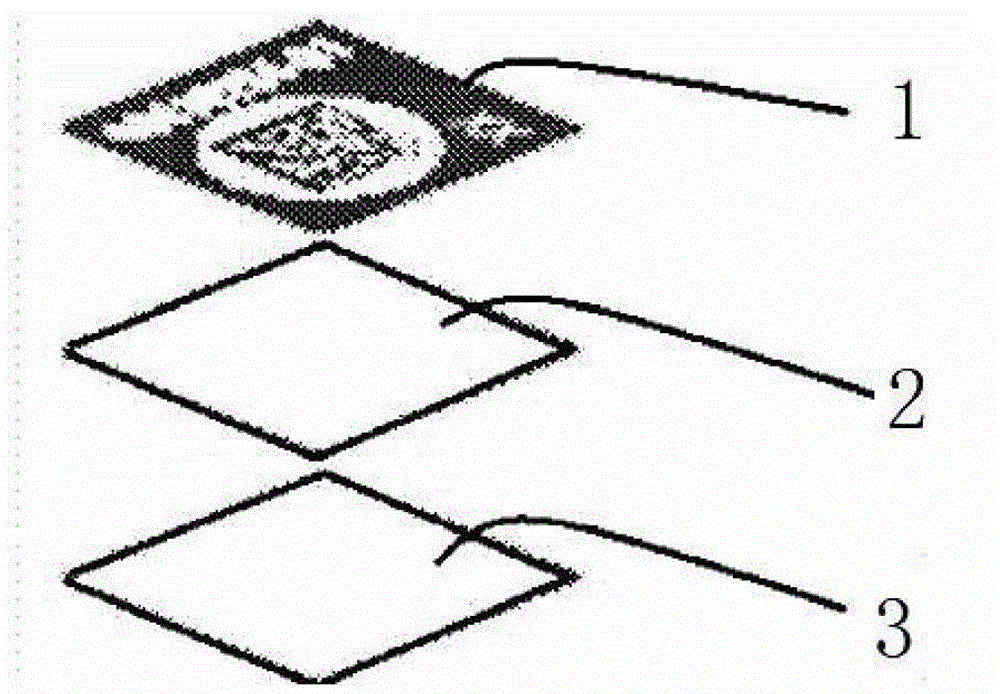
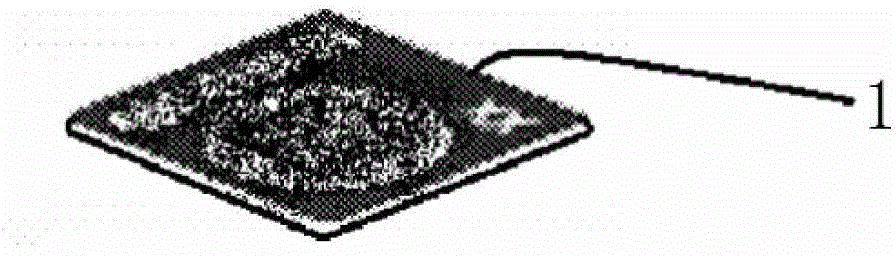
Vaccine failure indication label based on color change caused by temperature and preparation method thereof
A vaccine and indicator technology, applied in chemical instruments and methods, material analysis by observing the impact on chemical indicators, thermometers, etc., can solve the problems of vaccines that cannot be used and cannot be identified.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 1. Preparation of microcapsules
[0027] (1) Preparation of emulsion:
[0028] Preparation of sodium alginate solution: Take 90 parts of water, put it in a three-necked flask, and add 10 parts of sodium alginate solid evenly while stirring under the condition of a constant temperature water bath at a temperature of 30~60°C to fully dissolve it, and obtain 10% sodium alginate solution.
[0029] Preparation of monoglyceride solution: Take 1 part of monoglyceride solid, add 99 parts of water, stir to fully dissolve it in a constant temperature water bath at a temperature of 90°C, and obtain a monoglyceride solution with a mass fraction of 1%.
[0030] Mix 2.5 parts of monoglyceride solution, 5 parts of dodecyl alcohol or decanyl alcohol, 0.5 parts of Tween-80 and 92 parts of sodium alginate solution, and stir for 20 minutes at a rotational speed of 1500 r / min to obtain Lotion required.
[0031] (2) Preparation of coagulation bath
[0032] Get 5 parts of chitosan and 5 ...
Embodiment 2
[0042] 1. Preparation of microcapsules
[0043] (1) Preparation of emulsion:
[0044] Preparation of sodium alginate solution: Take 95 parts of water, put it in a three-necked flask, and add 5 parts of sodium alginate solid evenly while stirring under the condition of a constant temperature water bath at a temperature of 50°C to fully dissolve it to obtain the mass fraction 5% sodium alginate solution.
[0045] Preparation of monoglyceride solution: Take 6 parts of monoglyceride solid, add 94 parts of water, and stir to fully dissolve it in a constant temperature water bath at a temperature of 80°C to obtain a monoglyceride solution with a mass fraction of 6%.
[0046] Mix 6 parts of monoglyceride solution, 3 parts of dodecyl alcohol or decanyl alcohol, 0.5 part of Tween-80 and 90.5 parts of sodium alginate solution, and stir for 50 minutes at a rotating speed of 5000 r / min to obtain Lotion required.
[0047] (2) Preparation of coagulation bath
[0048]Get 2 parts of chito...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com