Biological sample pretreatment method, method for detecting rna, and pretreatment kit
一种生物样本、检测方法的技术,应用在生物化学设备和方法、微生物的测定/检验、DNA制备等方向
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
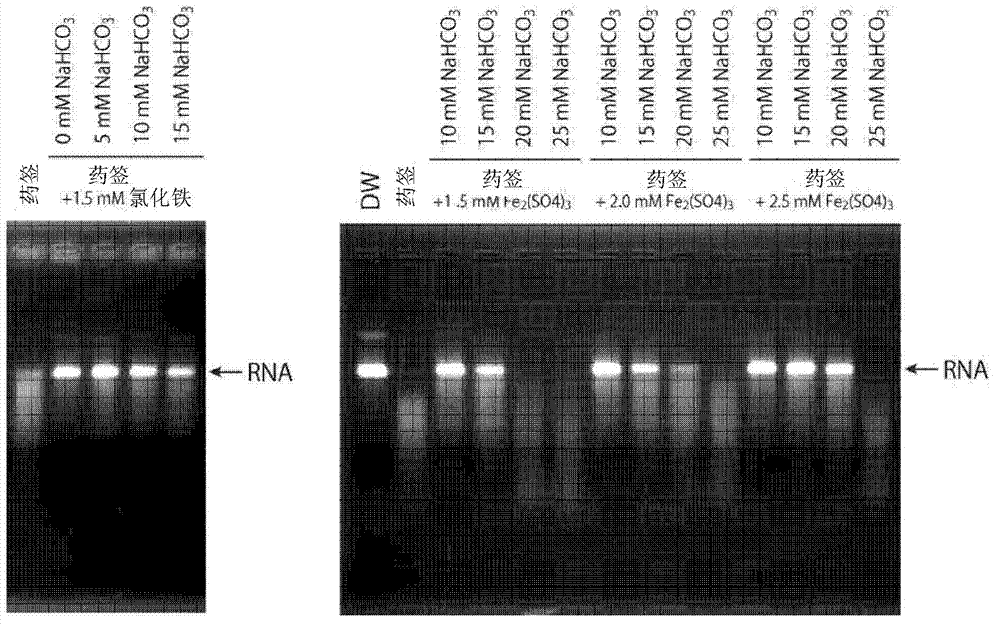
[0087] (Nucleic acid amplification of template RNA mixed in human nasal mucosa)
[0088] In this example, it was shown whether the pretreatment kit of the present invention can suppress the nucleic acid amplification inhibitory activity of template RNA derived from human nasal mucosa. The pretreatment kit was prepared to consist of 10 mM MES buffer (pH 5.8), 2.5 mM ferric sulfate, 20 mM sodium bicarbonate, 0.1% SDS, and 20 mg / ml activated alumina (manufactured by Wako Pure Chemical Industries, Ltd., molecular weight 101.96) Kit (pretreatment reagent). In this kit, the human nasal mucosa of a healthy person is suspended, and then, the template RNA having the same partial sequence as the virus (influenza A(H1N1)) prepared as follows is subjected to 10 7 Mixed replicates were used to make samples of this example. This sample was submitted to the RT-SmartAmp method to detect the presence of RNA. In addition, in the comparative example, except that iron sulfate and sodium bicarb...
reference example 1
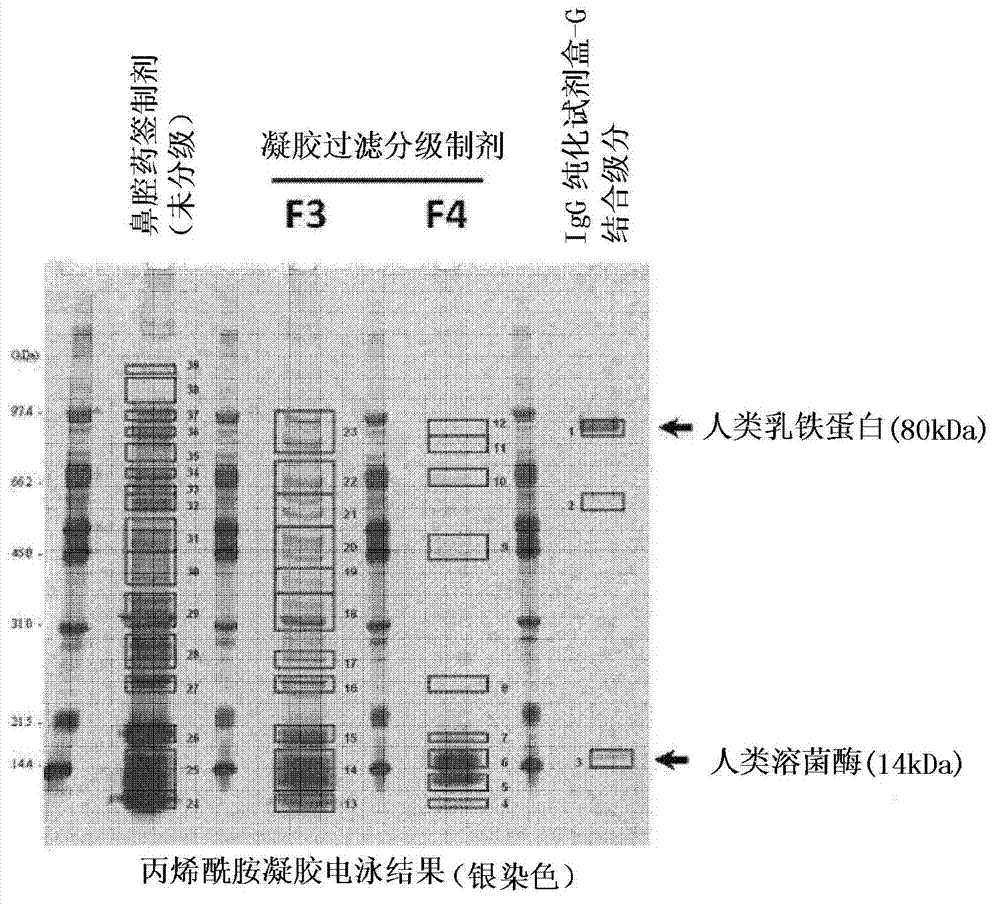
[0110] (presence of lactoferrin and lysozyme C in human nasal mucosa)
[0111] Such as figure 2 As shown in the electrophoresis photograph of , the presence of lactoferrin and lysozyme C was confirmed in human nasal mucosa. In addition, the presence of lactoferrin and lysozyme C in human nasal mucosa was confirmed as follows.
[0112] Nasal mucosa samples (mucosa of the upper throat) of healthy individuals were collected with a swab (MEN-TIP, manufactured by Nippon Cotton Swab Co., Ltd.), and each swab was suspended in 300 μL of phosphate buffered solution (PBS). The obtained suspension was subjected to gel filtration chromatography (phosphate buffer mobile phase, column HiLoad 16 / 60 Superdex 200 pg, manufactured by GE Healthcare), and size-selected into four fractions (F1 to F4). In addition, this suspension was applied to a protein G affinity spin column (manufactured by Dojin Chemical Co., Ltd.) to obtain a column-bound fraction. The gel filtration screening preparation...
Embodiment 2
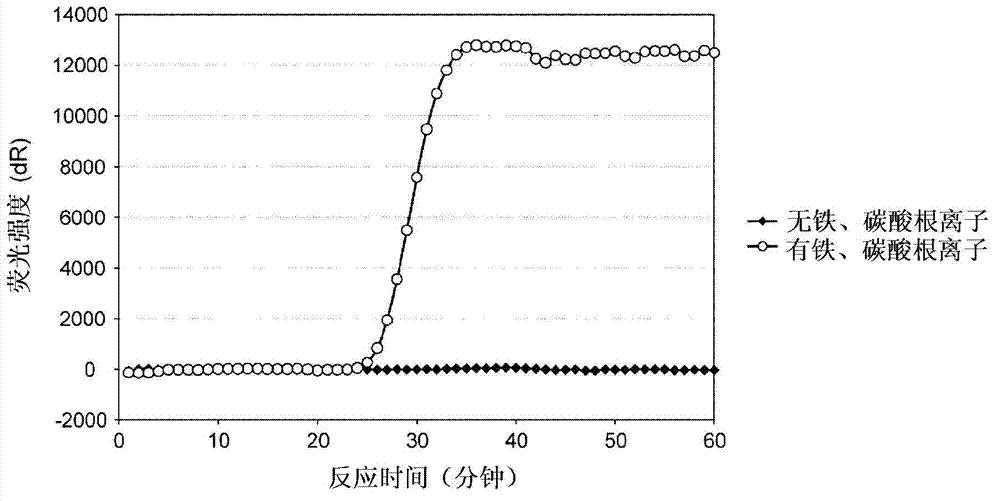
[0114] (Inhibitory effect of iron ions and carbonate ions on the RNA decomposition activity of human lactoferrin)
[0115] Such as image 3 As shown, the inhibitory effect of iron ions and carbonate ions on the RNA degradation activity of human lactoferrin was confirmed.
[0116] In this example, the same RNA as the synthesized template RNA was used as a substrate for RNA degradation activity (RNase activity). image 3 In the left panel, a nasal mucosa sample (mucosal membrane of the upper throat) of a healthy person was collected by a swab (MEN-TIP, manufactured by Japanese cotton swabs) and suspended in 250 μL of 40 mM Tris-HCl (pH 7.5). Next, 1.5 mM ferric chloride was added to 9 μL of the suspension, followed by the addition of 5 mM, 10 mM or 15 mM NaHCO 3 solution, the final volume was 18 μL. Then, the suspension was incubated at 37°C for 10 minutes. To its 2.5 μL suspension, 10 μL of 50 ng / μL RNA was added and incubated at 25° C. for 10 minutes. After the reaction, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com