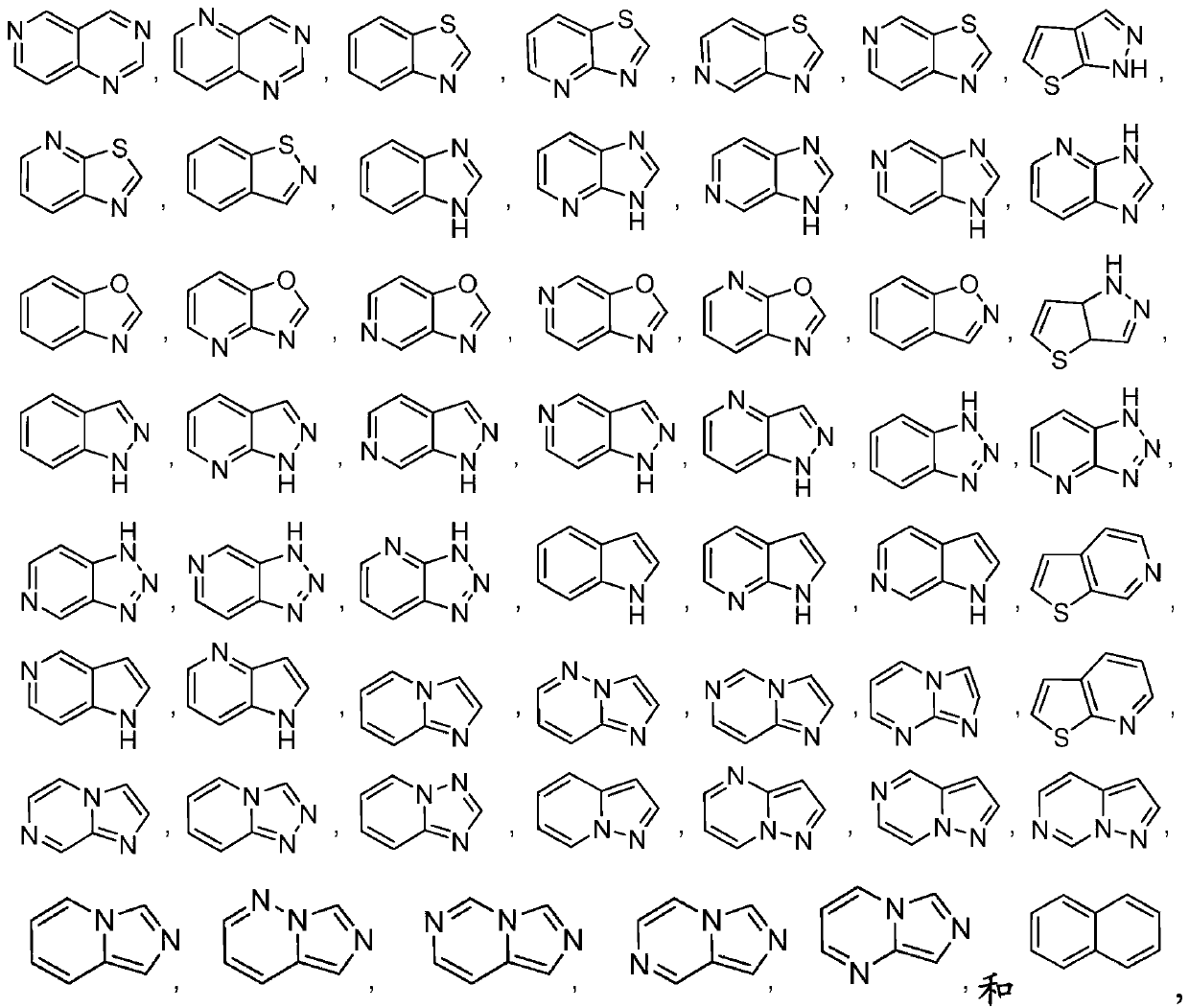
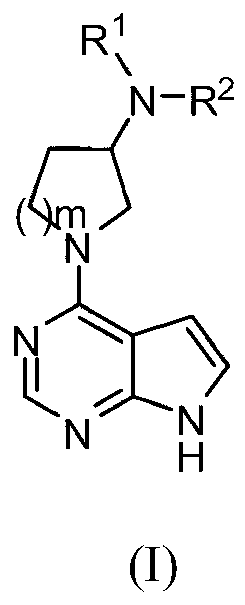
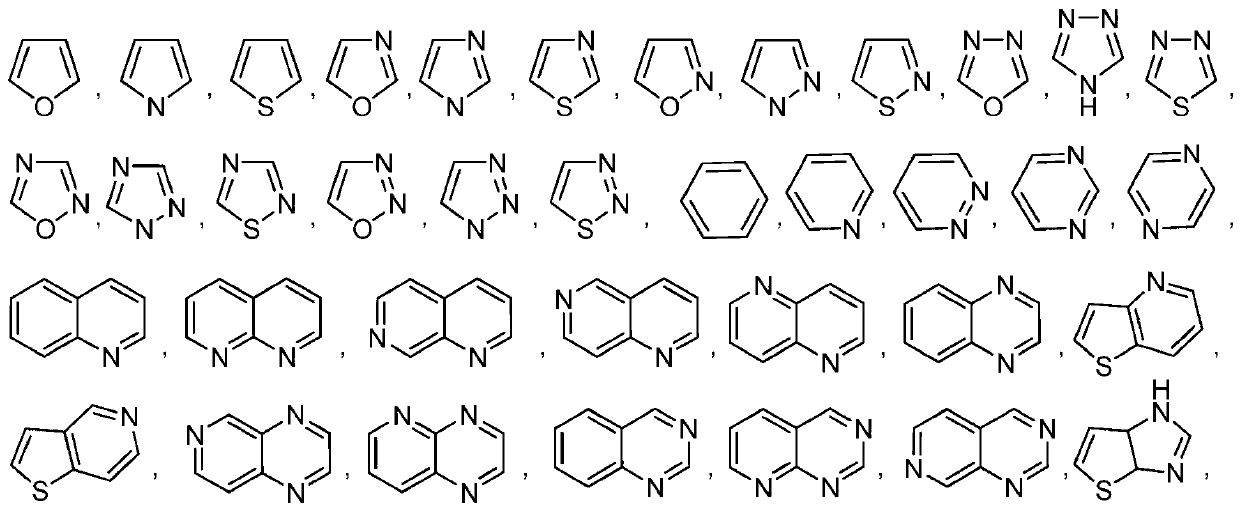
Pyrrolopyrimidine compounds and uses thereof
A compound, selected technology, applied in the fields of organic chemistry, drug combination, organic chemistry methods, etc., can solve problems such as unknown genetic basis of disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0281] Embodiment 1: the synthesis of compound 1-260
[0282] Compound 1
[0283] (R)-N-(1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)pyrrolidin-3-yl)-2-cyano-N-methylacetamide
[0284]
[0285] To (R)-N-methyl-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)pyrrolidin-3-amine (75mg, 0.345mmol) and 2-cyanoacetic acid (35mg , 0.414 mmol) in THF (5 mL) were added HATU (157 mg, 0.414 mmol) and DIPEA (0.12 mL, 0.69 mmol). The reaction mixture was stirred at room temperature for 20 hours. The precipitate was filtered, washed with EtOAc and dried under reduced pressure to give the title compound (45 mg, 46%). MS(m / z):285(M+H) + .
[0286] The following compounds were prepared according to the operation of Compound 1 using corresponding intermediates and reagents under suitable conditions recognized by those skilled in the art.
[0287]
[0288]
[0289] Compound 9
[0290] (R)-N-(1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)pyrrolidin-3-yl)-4-cyano-N-methylbenzenesulfonamide
[0291]
[0292] To...
Embodiment 2
[0540] JAK1 / 2 / 3 kinase assays were performed in vitro with Kit-Tyr6 Peptide (Invitrogen, cat# PV4122). With Z'-LYTE TM The Kinase Assay Kit-Tyr3Peptide (Invitrogen, Cat# PV3192) was used to perform the TYK2 kinase assay in vitro. Recombinant human JAK1 / 2 / 3 or TYK2 catalytic domains were from Invitrogen (catalogue # PV4774 / PV4210 / PV3855 / PV4790); all reactions (20 μL) were initiated by adding: 2.5 μL of test compound in 4% DMSO solution, 5 μL kinase / peptide substrate mix (3.2, 0.04, 0.2 or 8 μg / mL for recombinant human JAK1 / 2 / 3 catalytic domain, for Z-LYTE TM Tyr6Peptide or Z-LYTE TM Tyr3Peptide is 4 μM) or Phospho-Peptide solution (Invitrogen, catalog number PV3192, diluted with 1.33x kinase buffer), 2.5 μL ATP solution (300 / 100 / 40 / 100 μM, JAK1 / JAK2 / JAK3 / TYK2) or 1.33 x Kinase Buffer (Invitrogen, catalog number PV3189, diluted 5-fold with distilled water). A 384-well assay plate (Corning, Cat# 3575) was mixed and incubated for 1 hour at room temperatu...
Embodiment 3
[0543] Cytometry
[0544] To measure IL-6-induced STAT3 phosphorylation, HepG2 cells (SIBS) were cultured in serum-free DMEM medium at 5.4×10 3 cells / well were seeded overnight in 96-well plates at 37°C, 5% CO in the presence or absence of various concentrations of diluted compounds. 2 Incubate for 30 minutes. At 37°C, 5% CO 2 Next, cells were stimulated for 15 minutes by adding 10 ng / ml human recombinant IL-6 to each well. Cells were then fixed with 2% paraformaldehyde for 45 minutes at room temperature and incubated in ice-cold methanol for 30 minutes. After washing with PBS, cells were incubated overnight at 4°C with rabbit anti-phospho-STAT3 (Y705) (Cell Signaling Technologies, 1:1000, in antibody dilution solution) primary antibody. Goat anti-rabbit IgG Alexa488 (Invitrogen, 1:1000 dilution in PBS) secondary antibody was added and washed with PBS. Cells were counted after incubating in 7.5 uM propidium iodide, 100 μg / ml RNaseA, and PBS solution for 60 minutes in the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com