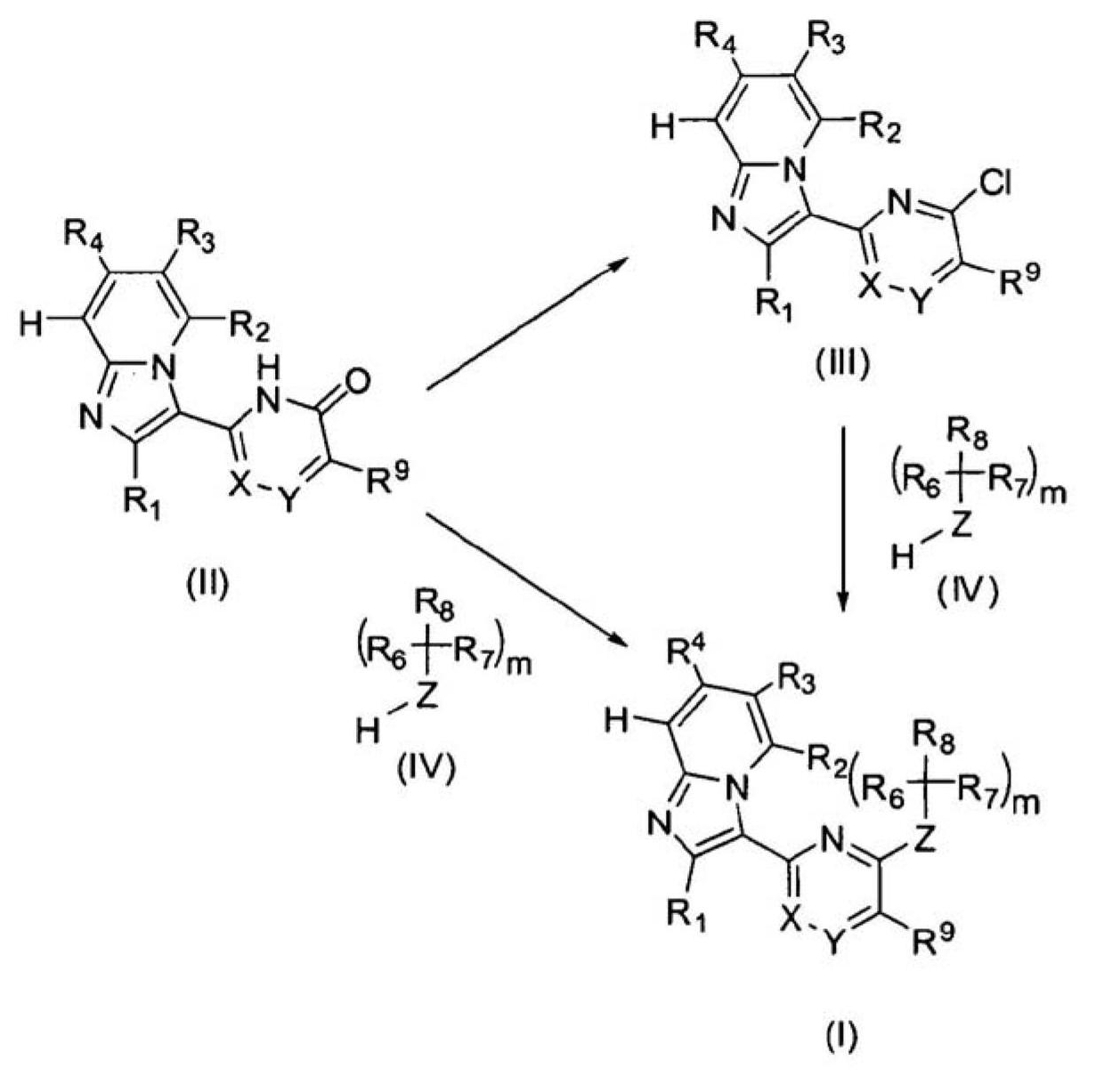
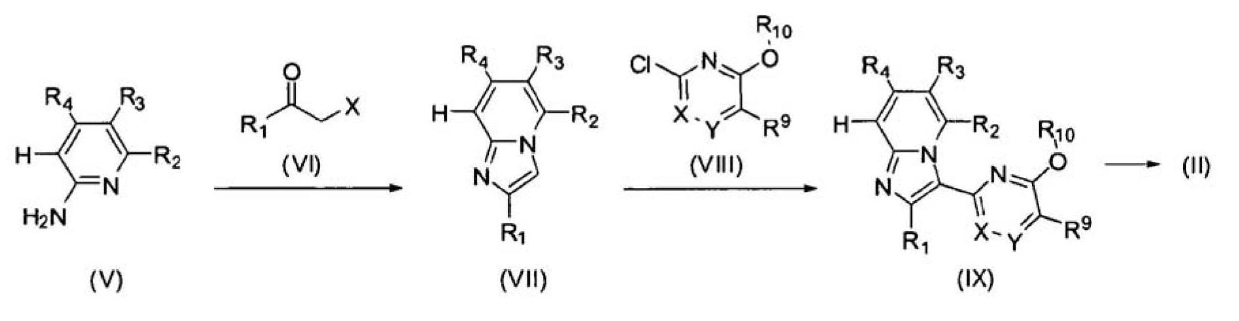
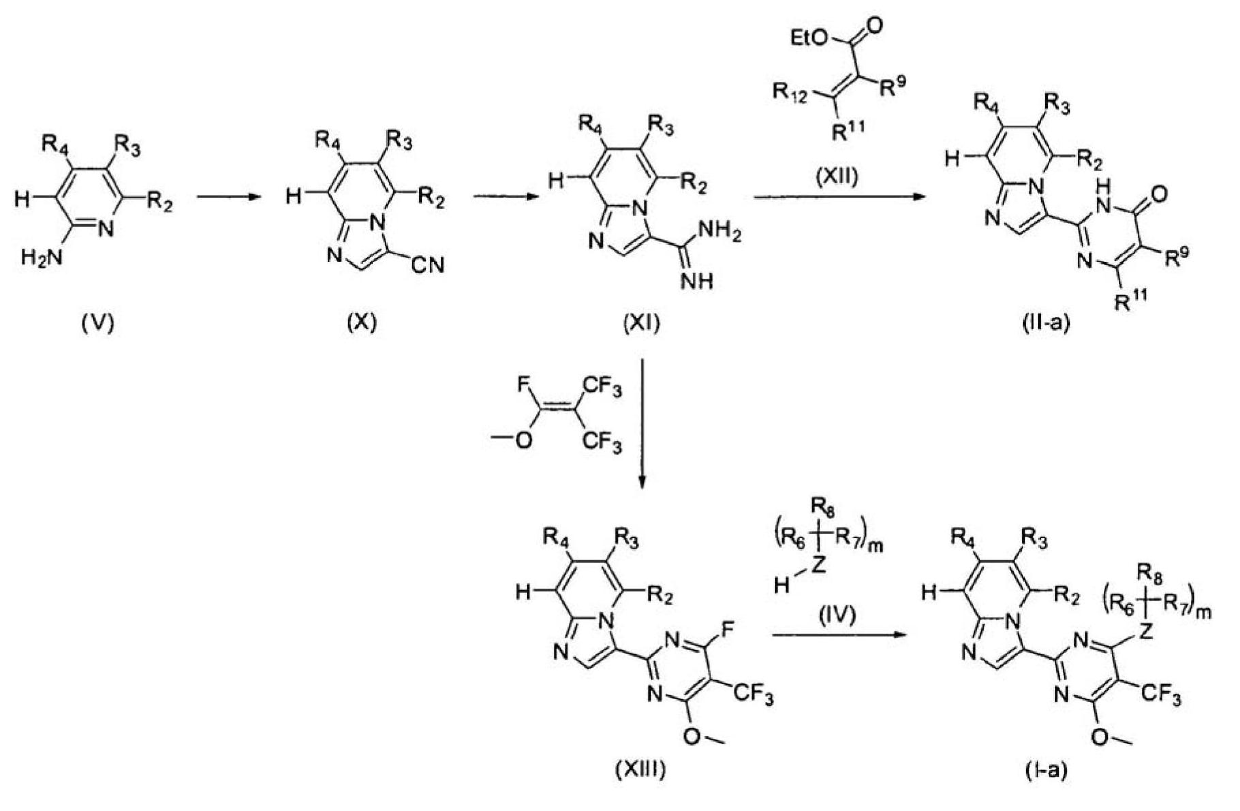
Imidazopyridine derivatives as JAK inhibitors
A technology of solvates and compounds, applied in the field of imidazopyridine derivatives as JAK inhibitors, can solve problems such as disorders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1717] 3-(4-{[(1S)-1-phenylethyl]amino}pyrimidin-2-yl)imidazo[1,2-a]pyridine-6-carbonitrile
[1718]
[1719] From 3-(4-hydroxypyrimidin-2-yl)imidazo[1,2-a]pyridine-6-carbonitrile (Preparation 4b, 0.175 g, 0.74 mmol) following the experimental procedure as described in Preparation 5a And (S)-1-phenyl-ethylamine (0.48 mL, 3.68 mmol) gave a yellow solid (0.102 g, 40%). The solvent was removed in vacuo and analyzed by reverse phase chromatography (C-18 silica from Waters, water / 1:1 acetonitrile-methanol as eluent [buffered with 0.1% v / v formic acid] 0% to 100%) to give the title compound (0.102 g, 40%) as a yellow solid.
[1720] LRMS(m / z):341(M+1) + .
[1721] 1 H-NMRδ(DMSO-d 6 ):1.53(d,3H),5.28(bs,1H),6.52(bs,1H),7.22(d,1H),7.36(d,2H),7.48(d,2H),7.67(d,1H) , 7.87 (d, 1H), 8.19 (d, 1H), 8.26 (bs, 1H), 8.42 (bs, 1H), 10.27 (bs, 1H).
Embodiment 2
[1723] 3-(4-{[(1R)-1-phenylethyl]amino}pyrimidin-2-yl)imidazo[1,2-a]pyridine-6-carbonitrile
[1724]
[1725] Following the experimental procedure as described in Preparation 5a, from 3-(4-hydroxypyrimidin-2-yl)imidazo[1,2-a]pyridine-6-carbonitrile (Preparation 4b) and (R)-1- Phenyl-ethylamine was obtained as a white solid (70%). After completion of the reaction, the mixture was partitioned between water and dichloromethane. The organic layer was washed with water, brine, and dried (MgSO 4 ) and evaporated, the residue was purified by flash chromatography (dichloromethane / methanol 98:2 to 96:4).
[1726] LRMS(m / z):341(M+1) + .
[1727] 1 H-NMRδ(DMSO-d 6 ):1.52(d,3H),5.30(bs,1H),6.52(d,1H),7.22(d,1H),7.34(t,2H),7.48(d,2H),7.68(d,1H) ,7.88(d,1H),8.19(d,1H),8.24(d,1H),8.43(s,1H),10.28(bs,1H)
Embodiment 3
[1729] 3-[4-(Benzylamino)pyrimidin-2-yl]imidazo[1,2-a]pyridine-6-carbonitrile
[1730]
[1731] Following the experimental procedure as described in Preparation 5a, yellow color was obtained from 3-(4-hydroxypyrimidin-2-yl)imidazo[1,2-a]pyridine-6-carbonitrile (Preparation 4b) and phenylmethylamine Solids (60%). Reaction crude material was purified by reverse phase chromatography (C-18 silica from Waters, water / 1:1 acetonitrile-methanol as eluent [buffered with 0.1% v / v formic acid] 0% to 100%) After that, the desired compound is obtained.
[1732] LRMS(m / z):327(M+1) + .
[1733] 1 H-NMRδ(DMSO-d 6 ):5.76(bs,2H),6.61(bs,1H),7.25-7.4(m,5H),7.68(d,1H),7.89(d,1H),8.17-8.36(m,2H),8.48( s,1H),10.37(bs,1H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com