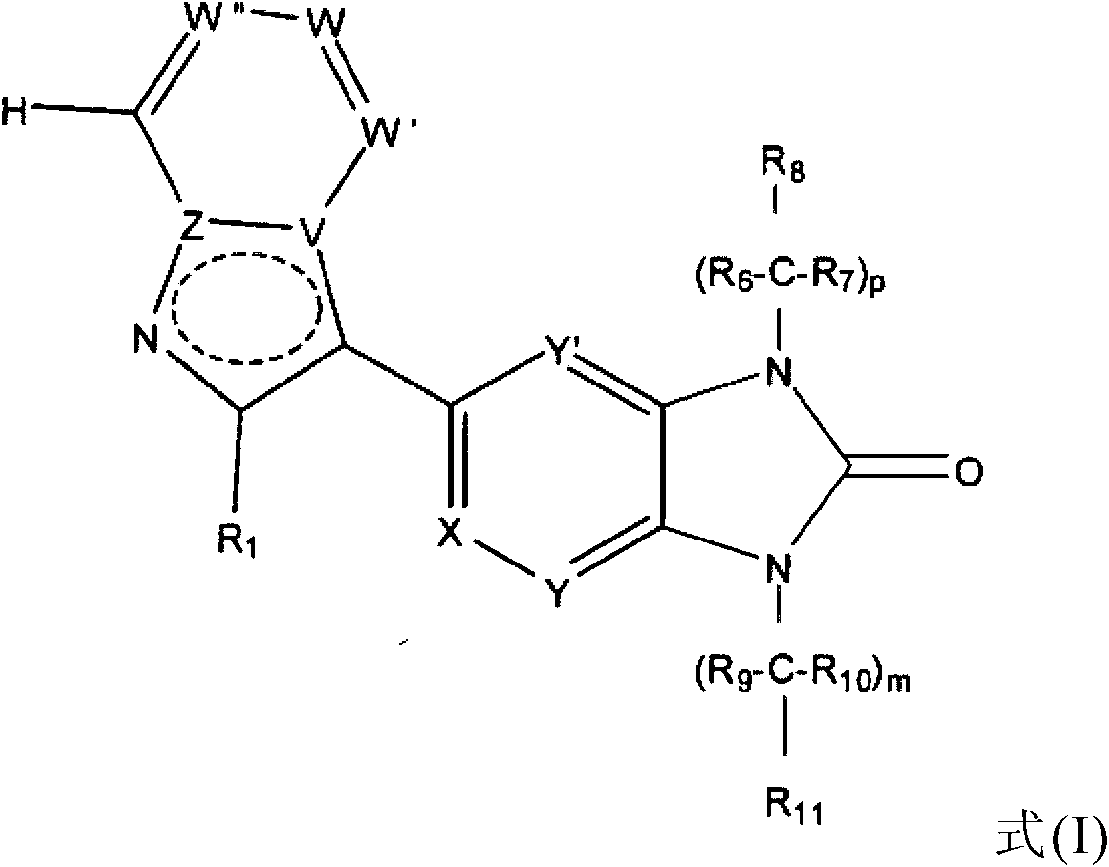
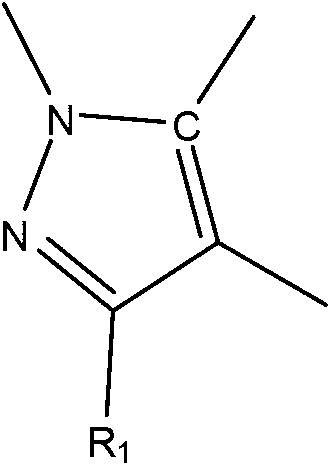
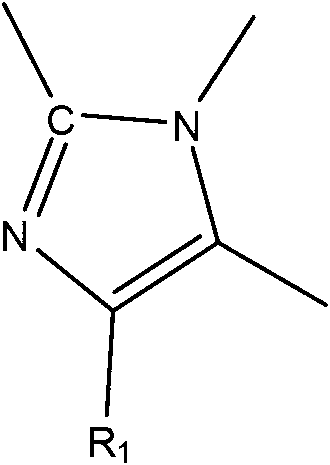
Heteroaryl imidazolone derivatives as JAK inhibitors
A technology of heterocyclic group and solvate is applied in the field of heteroarylimidazolone derivatives as JAK inhibitors, which can solve the problems of abnormal lymphoid tissue proliferation and reduction of thymus gland size.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0724] imidazo[1,2-a]pyridine-6-carbonitrile
[0725]
[0726]A 50% aqueous solution of 2-chloroacetaldehyde (26.40 mL, 210 mmol) was added to a solution of 2-aminonicotinonitrile (10 g, 80 mmol) in acetonitrile (300 mL), and the resulting mixture was stirred and heated to reflux temperature. After 20 hours, saturated aqueous sodium bicarbonate solution was added to partially saturate the resulting mixture, and additional saturated aqueous sodium bicarbonate solution was added until pH 7 was reached. The mixture was extracted with dichloromethane, and the organic layer was dried (MgSO 4 ) and evaporated, the residue was triturated with diethyl ether to give the title compound (10.75 g, 89%) as a brown solid.
[0727] LRMS(m / z):144(M+1) + .
[0728] 1 H NMR (300MHz, DMSO-d 6 ) δ ppm 7.49 (dd, 1H), 7.72 (s, 1H), 7.75-7.78 (m, 1H), 8.07 (s, 1H), 9.37 (s, 1H).
preparation example 2
[0730] 2-Chloro-9-(tetrahydro-2H-pyran-4-yl)-7,9-dihydro-8H-purin-8-one
[0731]
[0732] a) 2-chloro-5-nitro-N-(tetrahydro-2H-pyran-4-yl)pyrimidin-4-amine
[0733] Under nitrogen atmosphere, diisopropylethylamine (19.80 mL, 110 mmol) was added dropwise to stirred 2,4-dichloro-5-nitropyrimidine (11.56 g, 60 mmol) at -78 °C over 15 minutes and tetrahydro-2H-pyran-4-amine hydrochloride (prepared as described in WO200424728-A2, 7.81 g, 60 mmol) in dichloromethane (400 mL). The reaction mixture was stirred at -78°C for 2 hours and then allowed to warm to room temperature. The solvent was evaporated, water was added and the resulting solid was filtered, washed with water and dried to give the title compound (13.62 g, 93%) as a yellow solid.
[0734] LRMS(m / z):259(M+1) + .
[0735] 1 H NMR (300MHz, CDCl 3 ( s, 1H).
[0736] b) 2-Chloro-N 4 -(tetrahydro-2H-pyran-4-yl)pyrimidine-4,5-diamine
[0737] Zinc bromide (2.37g, 10.5mmol) and 5% platinum on carbon (5.13g, 25.7mmol...
preparation example 3
[0745] 2-Chloro-9-(tetrahydro-2H-pyran-4-yl)-7-{[2-(trimethylsilyl)ethoxy]methyl}-7,9-dihydro-8H- Purin-8-one
[0746]
[0747] Sodium hydride (60% dispersion in mineral oil, 0.40 g, 10.0 mmol) was added portionwise to the stirred 2-chloro-9-(tetrahydro-2H-pyran- 4-yl)-7,9-dihydro-8H-purin-8-one (Preparation Example 2, 1.98g, 7.8mmol) in N,N'-dimethylformamide (30mL) solution. After 15 minutes, trimethylsilane (1.53 mL, 8.6 mmol) was added and the mixture was warmed to room temperature and stirred for 4 hours. The mixture was separated into an aqueous layer and an ethyl acetate layer, and the organic layer was washed with water and brine, dried (MgSO 4 ) and evaporate the solvent under reduced pressure. The residue was purified by flash chromatography (99:1 dichloromethane / methanol) to afford the title compound (2.94 g, 98%) as a pale yellow oily residue.
[0748] LRMS(m / z):385(M+1) + .
[0749] 1 H NMR (300MHz, CDCl 3 )δppm-0.20-0.08(m, 9H), 0.92(m, 2H), 1.73(m, 2H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com