Anti-tie2 antibody and use thereof
A TIE-2 and antibody technology, applied in the direction of antibodies, antibody medical components, applications, etc., can solve the problems of reduced drug accessibility and reduced therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0122] Example 1 Screening of Antibodies Binding to TIE2
[0123] A human primary ScFv (scFv) library disclosed in Korean Patent Laid-Open Publication No. 10-2008-0109417 was used to screen for antibodies binding to TIE2 and to prepare the library. Spread 100 μl of antigen (hTIE2-his: Sino Biological, 10700-H08H) on 96-well Ni at 2 μl / ml + plate and incubate overnight at 4°C. The next day, the antigen-coated plate was washed three times with 0.1% TBST, and then reacted with 200 μl of 2% BSA blocking buffer for 2 hours at room temperature. Add 50 μl of XL1-Blue stock solution to 2 ml of 2xYT-TET (tetracycline 10 μg / ml) growth medium, and incubate at 37°C at 200 rpm for 2 hours, then add 13 ml of 2xYT-TET growth medium, and Incubate until OD 600 reach 0.5. After blocking for 2 hours, the wells were washed 3 times with 1XPBS. The phage library group was combined with each washed well, the phage library was mixed with 4% BSA in equal amounts, and then 200 µl of the mixture wa...
Embodiment 2
[0124] Example 2 Screening of monoclonal ScFv phage that specifically binds to TIE2 and neutralizes the binding to TIE2 (combination ELISA)
[0125] After completing the panning process, the final circular cell stock solution was diluted to form 200 to 500 colonies, plated on CM agar plates, and then incubated overnight at 37 °C. The next day, when the colony grows, add 200 μl of 2xYT medium (CM 34 μg / ml+1% glucose) into a 96-well deep-well plate, insert one colony into each well, and then place the plate at 37°C and 3,000rpm Incubate overnight. The next day, spread 200 μl of 2xYT medium (CM 34 μg / ml+1% glucose) on a fresh 96-well deep-well plate, inject 20 ml of the cells grown the day before into each well, and incubate at 37°C and 3,000 Grow for 1 hour and 10 minutes at rpm. Store the remaining cells in 100 μl of 50% glycerol at -70 °C. While the cells were growing, mix 1 μl of helper phage with 19 μl of 2xYT medium, inject 20 μl of the resulting mixture into each well, ...
Embodiment 3
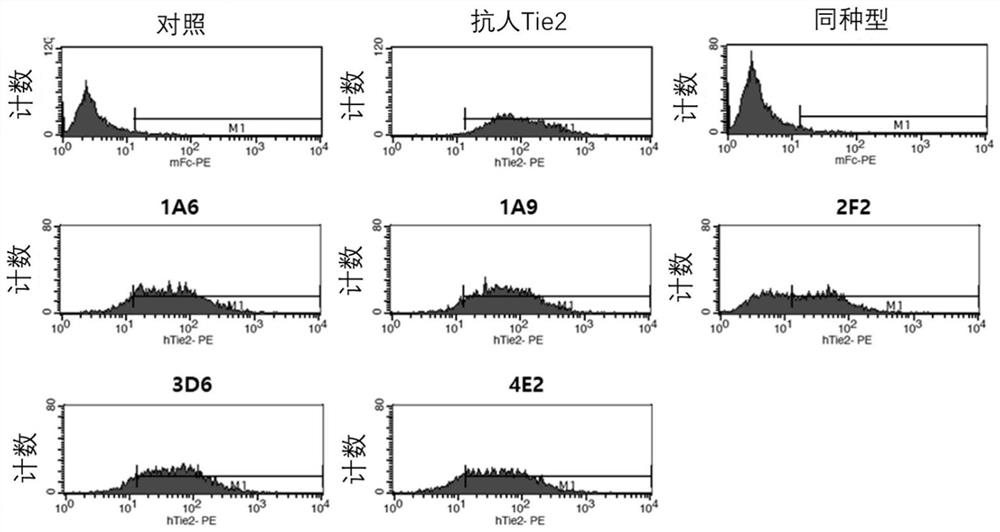
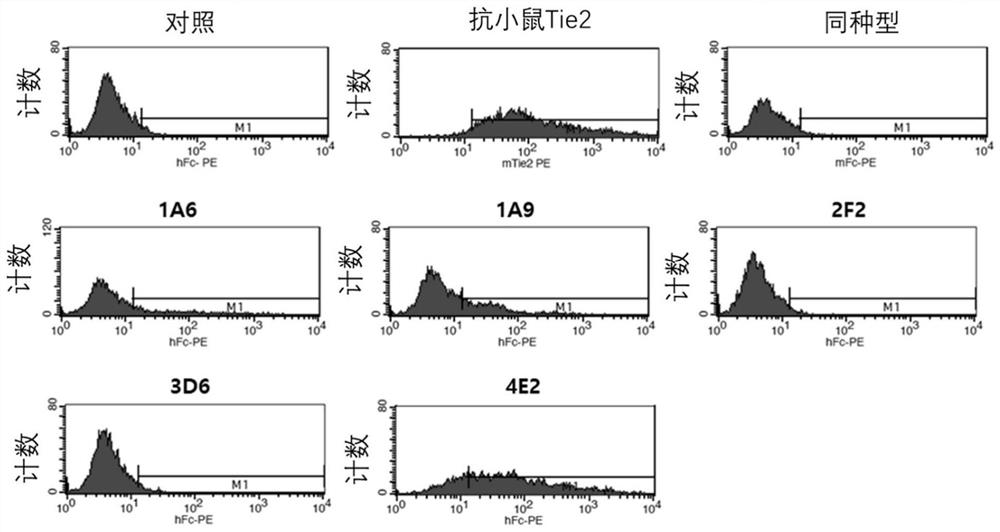
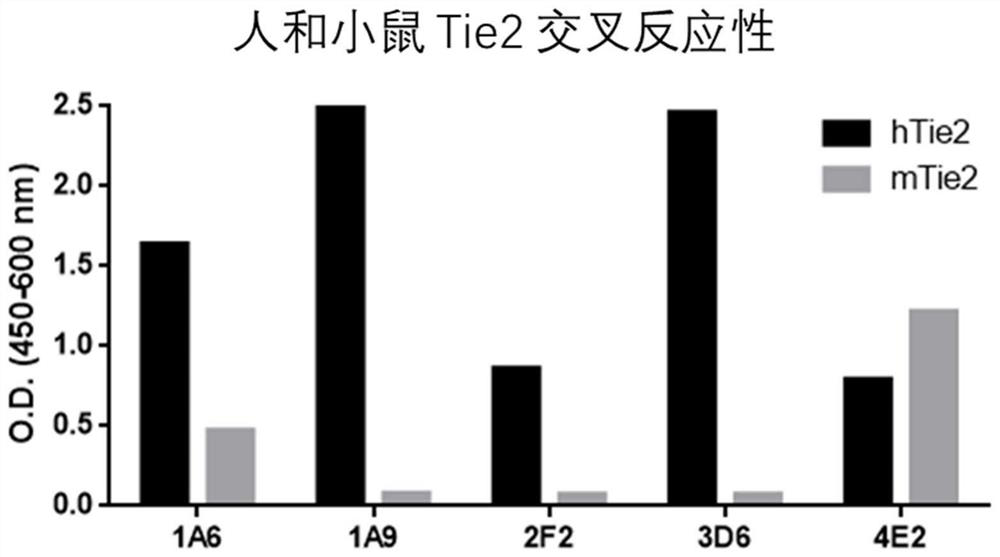
[0141] Example 3 Selection of antibodies with binding ability to cells expressing TIE2
[0142] To determine binding to TIE2-expressing cells, binding to self-established human / mouse TIE2-overexpressing CHO-K1 cell lines (hTIE2 / CHO-K1, mTIE2 / CHO-K1) was measured using flow cytometry. Each cell line was maintained and cultured, hTIE2 CHO-K1 was washed with PBS, 5 mM EDTA was added thereto to obtain a cell suspension, and each cell was recovered by centrifugation. Prepare 5×10 cells in FACS-specific buffer (PBS containing 2% FBS, 0.05% sodium azide) 6 cells / mL of cell suspension, and dispense 100 μL of cell suspension into each tube. Add 100 µL of 2 µg / mL clones to each tube and let stand at 4 °C for 30 min to induce cell binding. The cells were washed with 2 mL of FACS buffer, 100 μL of secondary antibody dilution (1 / 500, anti-human IgG-Fc polyclonal antibody fragment conjugated with PE, #A80-248PE, BethylLaboratories Inc.US) was added to it, and the resulting The mixture wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com