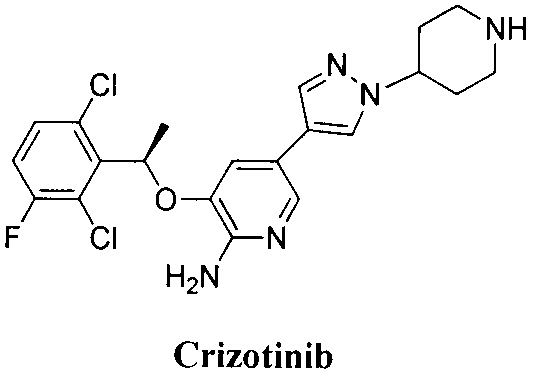
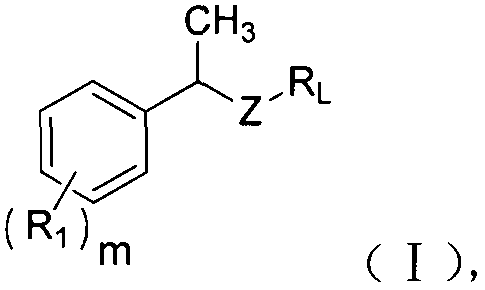
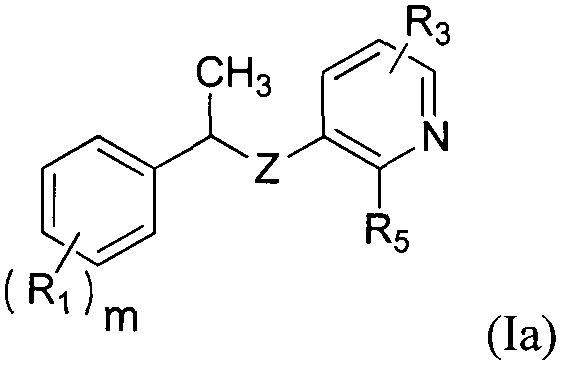
Novel tyrosine protein kinase inhibitor
A technology of amino and alkylamino, applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0119] Example 1 2-amino-N-(3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)pyridin-2-yl)propionamide
[0120]
[0121] Step A: 1-(2,6-Dichloro-3-fluorophenyl)ethanol
[0122] Under the condition of ice bath, add 20.6g of 2,6-dichloro-3-fluoroacetophenone and 50ml of methanol into a 250ml one-necked flask, slowly add NaBH 4 5.7g, after the addition, continue to react for 4h, evaporate methanol under reduced pressure to obtain an oil, add 20ml of water, adjust the pH to neutral with dilute hydrochloric acid, extract with ethyl acetate, dry over anhydrous sodium sulfate, filter, and precipitate under reduced pressure. The title compound was obtained.
[0123] Step B: 2-nitro-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)pyridine
[0124] Under a nitrogen atmosphere, add 0.23 g of the product from step A and 10 ml of THF to a 50 ml three-necked flask, then add 0.175 g of 2-nitro-3-hydroxypyridine and 0.44 g of triphenylphosphine, and stir at room temperature for 1 h. Diisopropyl azodi...
Embodiment 2
[0131] Example 2 2-amino-N-(3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1-(piperidin-4-yl)-1H- Pyrazol-4-yl)pyridin-2-yl)propionamide
[0132]
[0133] Step A 2-amino-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-bromopyridine
[0134] Under ice-bath conditions, add 20ml of acetonitrile to a 50ml three-neck flask, 0.7g of the product obtained in step C of Example 1, slowly add 0.4g of NBS, stir at room temperature for 2 hours, filter, wash the filter cake twice with acetonitrile, and obtain 0.7 g of the title compound.
[0135] Step B 2-amino-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1-(N-tert-butoxycarbonylpiperidin-4-yl)- 1H-pyrazol-4-yl)pyridine
[0136] Under the protection of nitrogen, add 3-(4-(4,4,5,5-tetramethyl-1,3-diketonylboronic acid pentane-2-yl)-1H-pyrazole-1 into a 50ml three-necked flask - Base) tert-butyl piperidine-1-carboxylate 0.25g, DME 10ml, step A product 0.2g, then add palladium catalyst 0.018g, cesium carbonate aqueous solution, reflux at 85°C...
Embodiment 3
[0141] Example 3 3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-2-(N-methylamino)pyridine
[0142]
[0143] Under the protection of nitrogen, add 0.5 g of the result of step C of Example 1 into a 50 ml three-necked bottle, dissolve it in THF, and add excess (BOC) at 25 ° C 2 O, stirred at 50°C for 24 hours, TLC monitored that the basic reaction of the raw materials was complete, desolvated under reduced pressure, added saturated sodium bicarbonate solution to adjust the pH to between 7-8, extracted with ethyl acetate, dried over anhydrous sodium sulfate, filtered, and reduced pressure The title compound was obtained by precipitation.
[0144] Under nitrogen protection, mix 0.5g of the above product, 0.48g of methyl iodide, 0.23g of potassium carbonate, and 10ml of DMF, and stir at 60°C for 6h. After the reaction is complete, pour the reaction solution into 100mL of saturated saline, and extract with ethyl acetate (20mL×3) , dried over anhydrous sodium sulfate, filtered, precipita...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com