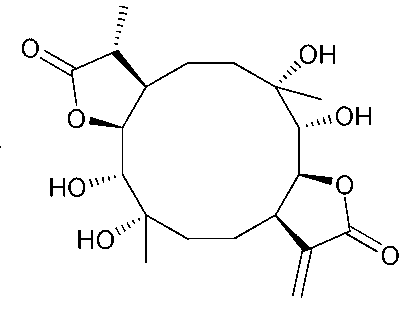
Application of Eryngiolide A in medicines curing yellow fever virus infection
A yellow fever virus and drug technology, applied in the field of biomedicine, to achieve the effect of effective prevention and treatment, outstanding substantive characteristics, huge social benefits and research value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: Preparation of the compound Eryngiolide A tablet involved in the present invention:
[0037] Take 20 grams of compound Eryngiolide A, add 180 grams of conventional excipients for tablet preparation, mix well, and make 1000 tablets with a conventional tabletting machine.
Embodiment 2
[0038] Embodiment 2: the preparation of the compound Eryngiolide A capsule of the present invention:
[0039] Get 20 grams of compound Eryngiolide A, add conventional auxiliary materials for preparing capsules such as 180 grams of starch, mix well, and pack into capsules to make 1000 tablets.
[0040] The following pharmacodynamic experiments will further illustrate its drug activity.
experiment example 1
[0042] A kind of application of Eryngiolide A in the preparation treatment or prevention yellow fever virus infection medicine, its steps are:
[0043] A. Toxicity test of Eryngiolide A on Vero cells
[0044] Vero cells (African green monkey kidney cells) are susceptible cells to YFV.
[0045] The experimental steps are as follows:
[0046] 1: Inoculate Vero cells: Use DMEM medium containing 10% (v / v) fetal bovine serum to make a single cell suspension, inoculate 1000-10000 cells per well into a 96-well cell culture plate, and inoculate a volume of 100ul per well ;
[0047] 2: Culture Vero cells: at 37°C, 5% (v / v) CO 2 Under culture conditions, cultivate for 2 days;
[0048] 3: Add Eryngiolide A: Discard the DMEM medium in each well, add 100ul to each well and dilute to the corresponding concentration with DMEM medium containing 10% (v / v) fetal bovine serum (0uM, 0.4uM, 1.2 uM, 3.7uM, 11uM, 33uM, 100uM, 300uM) of Eryngiolide A, add 100ul of DMEM medium containing 10% (v / v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com