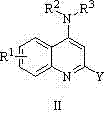
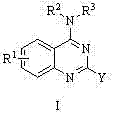
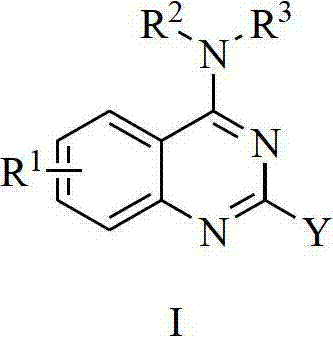
4-aminoquinoline and 4-aminoquinoline compound and applications of compound
A technology of aminoquinazolines and compounds, applied in the field of medicine, can solve problems such as undiscovered anti-tumor research reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0172]The examples are intended to illustrate, not limit, the scope of the invention. The H NMR spectra of the compounds were determined by Bruker ARX-300, and the mass spectra were determined by Agilent 1100 LC / MSD; the reagents used were analytical or chemically pure.
[0173] Ar 1 Preparation of CHO intermediate
[0174] Some aromatic aldehydes are commercially available; others are prepared as follows:
[0175] Method 1: Preparation of 1-substituted benzyl-indole-3-carbaldehydes
[0176]
[0177] Anhydrous potassium carbonate (0.11mol) and indole-3-carbaldehyde (0.1mol) were added to 60mL of DMF, stirred at room temperature for 20 minutes, and ArCH was added to the reaction solution 2 Cl (0.1 mol). After dropping, react at room temperature for 6 h, pour the reaction solution into 300 mL of water, stir for 0.5 h, filter with suction, wash with water, and dry to obtain 1-substituted benzyl-indole-3 carboxaldehyde.
[0178] Method 2: Preparation of 1-substituted benzy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com