Application of Erbin inhibitor in preparation of antitumor drug
A technology of anti-tumor drugs and inhibitors, which is applied in the application field of Erbin inhibitors in the preparation of anti-tumor drugs, and can solve the problems of easy recurrence and drug resistance of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
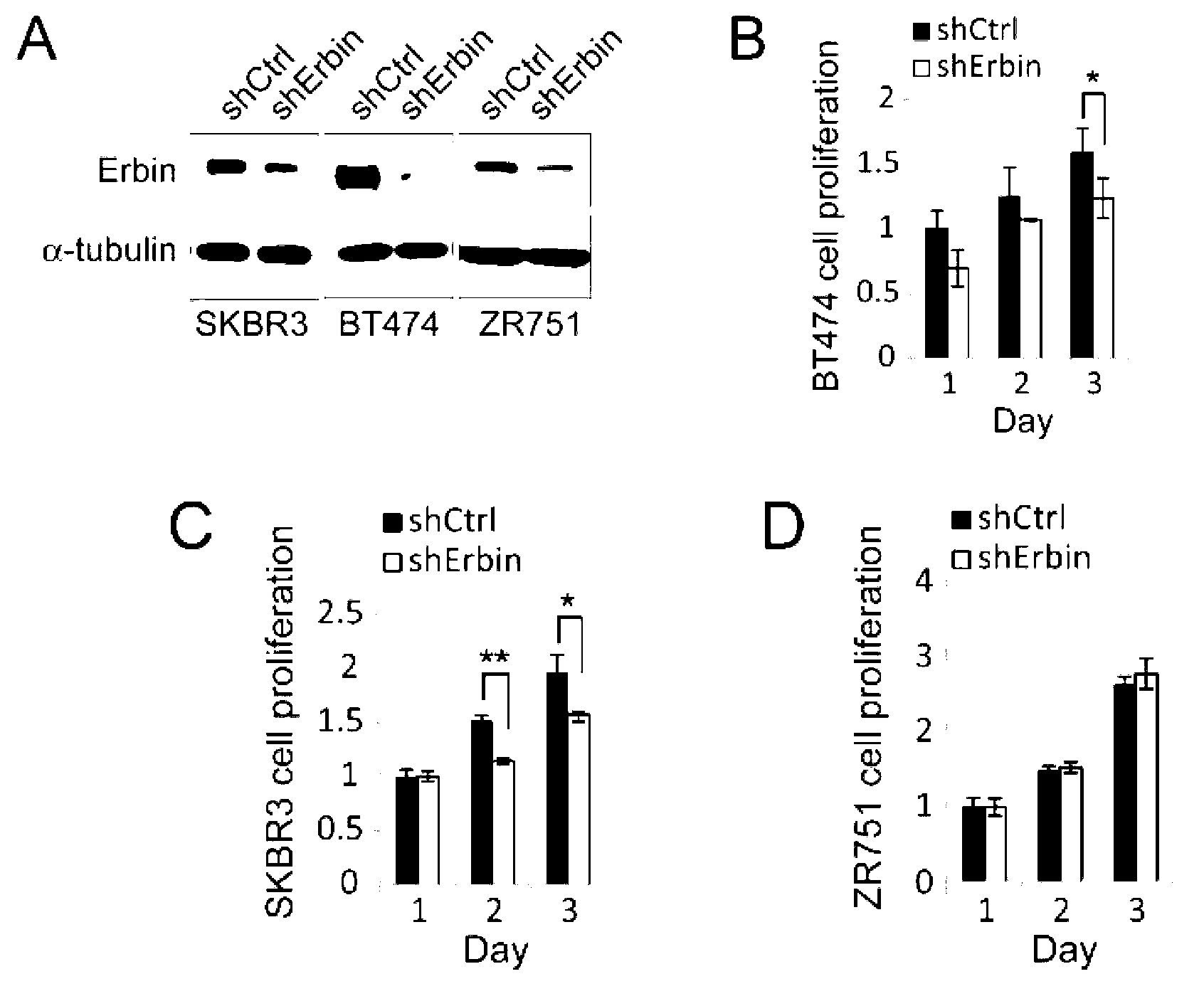
[0035] Example 1: Cell models and animal models are used to confirm that Erbin is extremely important to the generation and development of ErbB2-dependent breast cancer, providing a way and means to treat ErbB2-dependent tumors by knocking down the expression of Erbin with specific shRNA
[0036] To study the effect of Erbin on breast cancer cell proliferation, ErbB2-dependent breast cancer cell lines BT474 (ATCC) and SKBR3 (ATCC), and ErbB2-independent breast cancer cell line ZR751 (Public Health England) were selected. When these cells were cultured to full plate, shRNA specifically recognizing Erbin (shErbin) packaged in lentivirus (forward strand: 5'TGCAT CCCTC TAGAG AACAA CTTTC AAGAG AAGTT GTTCTCTAGA GGGAT GCTTT TTTC; reverse strand: 5'TCGAG AAAAA AGCAT CCCTCTAGAG AACAA CTTCT CTTGA AAGTT GTTCT CTAGA GGGAT GCA.) and another control shRNA (shCtrl) (pLL3.7 empty vector) respectively invaded the cells (the titer was 8x10 8 Add 1 μl of IU / ml virus particle suspension into a 10...
Embodiment 2
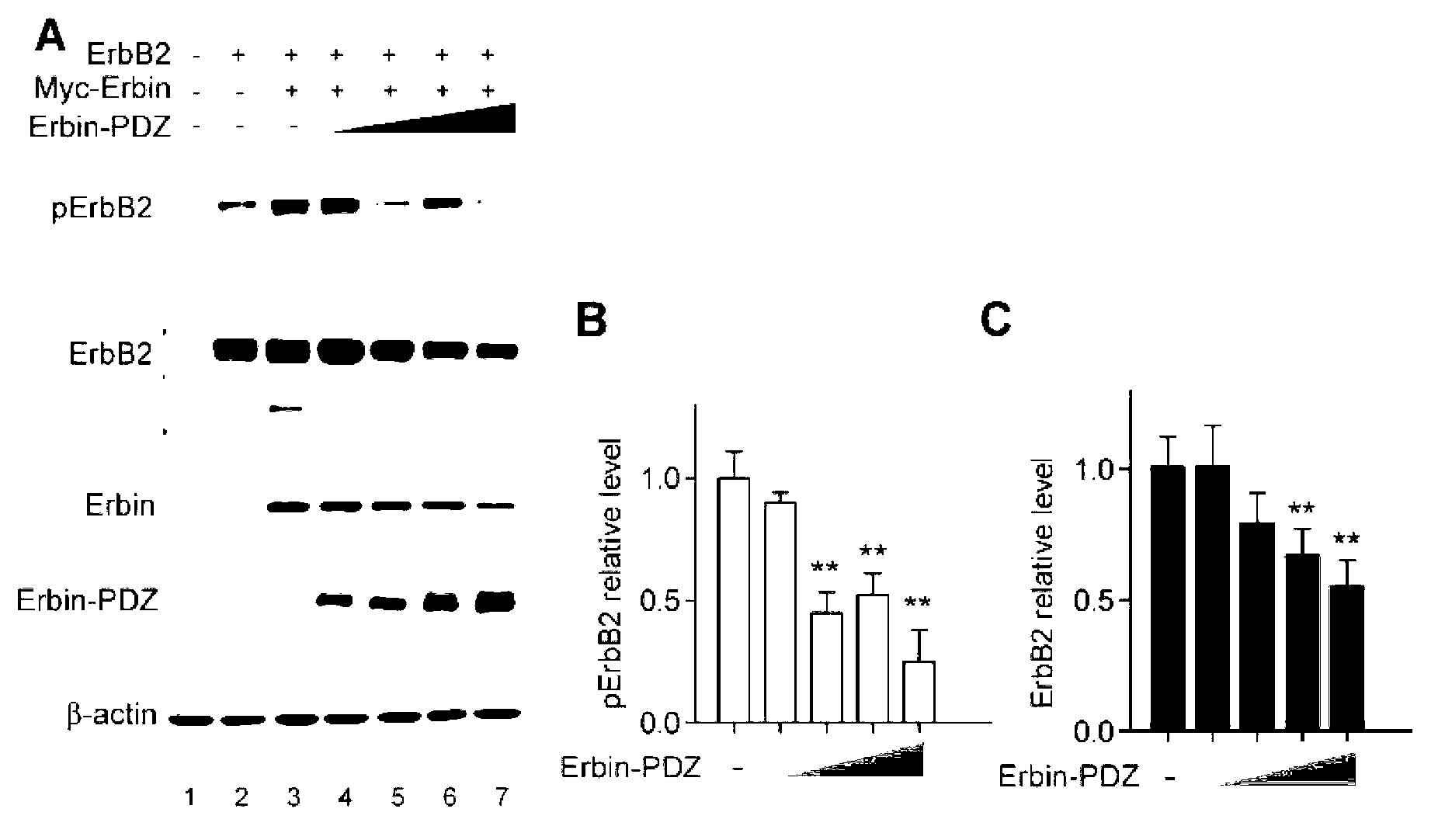
[0040] Example 2: Cell models and animal models were used to confirm that the PDZ structural fragment of Erbin is extremely critical for ErbB2-dependent breast cancer formation. Overexpression of the PDZ fragment of Erbin can interfere with the interaction of Erbin-ErbB2 to reduce the protein level of ErbB2 and its kinase activation, thus providing a therapeutic approach and means for treating ErbB2-dependent tumors by targeting the PDZ fragment of Erbin
[0041] Earlier work by the inventors has revealed that Erbin binds to ErbB2 through the PDZ structural region. The PDZ structural fragment at the C-terminus of Erbin and the PDZ-binding sequence at the C-terminus of ErbB2 are critical for their binding and interaction. To investigate whether the binding of Erbin to ErbB2 is important for Erbin to regulate the protein stability and function of ErbB2, a plasmid expressing only the PDZ structural fragment of Erbin was constructed
[0042] (ATGGAGATTCGAGTGAGGGTTGAAAAGGATCCAGAAC...
Embodiment 3
[0081]Example 3: Cell models and animal models confirm that the C-terminal polypeptide fragment of ErbB2 can interfere with Erbin-ErbB2 interaction to reduce the protein level and kinase activity of ErbB2 in cancer cells, providing an exogenous C-terminal of excessive ErbB2 Ways and means of treating tumors with polypeptide B2tail
[0082] In order to develop an easy-to-apply therapeutic approach targeting Erbin-ErbB2 interaction, a polypeptide fragment B2tail (PTAENPEYLGLDVPV) containing 15 amino acid residues at the end of ErbB2 was synthesized. Myc-Erbin and Flag-ErbB2 plasmids were transfected into HEK293 cells, and the cells were lysed one day after protein expression. Equal amounts of cell lysate containing Myc-Erbin and Flag-ErbB2 proteins were mixed with 200 μl of 50 μM B2tail polypeptide or unrelated polypeptide (Ctrl) and incubated for one hour, and then immunoprecipitated with anti-Myc antibody. The immunoprecipitated products were detected by Western blotting, and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com