High-stability compound chlorhexidine iodohydrin solution and preparation method thereof
A compound chlorhexidine iodine, high-stable technology, which can be used in pharmaceutical formulations, active ingredients of iodine compounds, and medical preparations of non-active ingredients, etc., can solve the problems of iodine loss, easy generation of drug-resistant bacteria, and increased synthesis cost. , to achieve the effect of reducing iodine loss, ingenious design and fast onset
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] 1.3 Preparation of highly stable compound chlorhexidine iodine alcohol solution
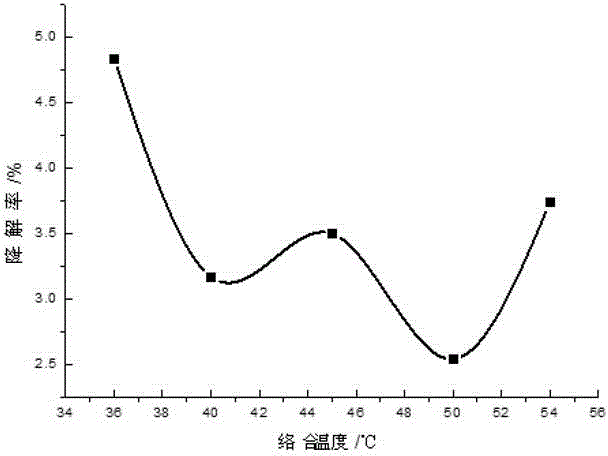
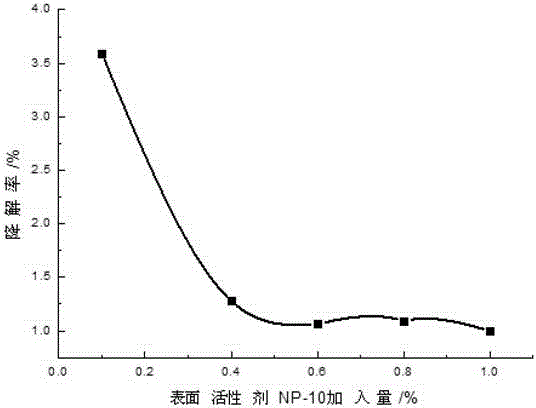
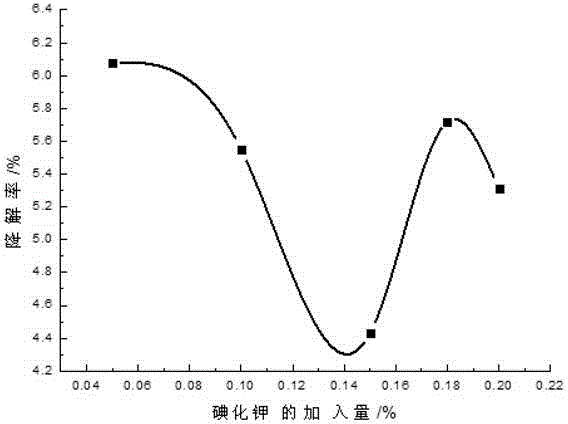
[0035] Add 0.1-2 parts by weight of 10% PVPI, 0.1-1 parts by weight of chlorhexidine acetate and 0.01-0.23 parts by weight of iodine into a three-necked flask containing 50-70 parts by weight of ethanol, and simultaneously add 0.01-0.3 parts by weight of KI , stirring and reacting for about 2-3 hours at 40-60° C. under sealed conditions, and then adding 0.1-2 parts by weight of surfactant and humectant and 29.36-42.69 parts by weight of water after cooling. Finally, adjust the pH to 2-4 with phosphoric acid.
[0036] 1.4 Determination of available iodine content
[0037] The available iodine concentration of the sample can be determined with reference to the potentiometric titration method in the Chinese Pharmacopoeia [2] method. In this experiment, the potentiometric titration method in the Chinese Pharmacopoeia was used to measure the effective iodine content: accurately pipette 25ml o...
Embodiment 1
[0080] Add 0.1 parts by weight of 10% PVPI, 0.1 parts by weight of chlorhexidine acetate and 0.23 parts by weight of iodine into a three-necked flask filled with 70 parts by weight of ethanol, and add 0.01 parts by weight of KI at the same time, and stir the reaction at 40°C under sealed conditions for 3 About hour, just add the tensio-active agent (0.01 weight part K30 of 0.09 weight part OB-2 and the humectant glycerin of 0.1 weight part after cooling, and the water of 29.36 weight parts respectively after cooling. Regulate pH with phosphoric acid at last to be 2 .
[0081] After testing, the sample was prepared according to the disinfection technical specifications and the requirements of the Chinese Pharmacopoeia. The available iodine was 0.19%. After the stability test was accelerated for 14 days, the degradation rate was 6.11%. It was applied on the arm, and the arm was dry after 2-3 minutes. No obvious yellowing phenomenon, easy to wash off with water. The average kill...
Embodiment 2
[0086] Add 1 part by weight of 10% PVPI, 0.51 parts by weight of chlorhexidine acetate and 0.13 parts by weight of iodine into a three-necked flask filled with 60 parts by weight of ethanol, and add 0.15 parts by weight of KI at the same time, and stir the reaction at 50 °C under sealed conditions for 2.5 About hours, after cooling, 1 part by weight of surfactant NP-10, 1 part by weight of humectant K30, and 36.21 parts by weight of water were added respectively. Finally, the pH was adjusted to 3 with phosphoric acid.
[0087] After testing, the sample was prepared according to the disinfection technical specifications and the requirements of the Chinese Pharmacopoeia. The available iodine was 0.2%. After the stability test was accelerated for 14 days, the degradation rate was 3.11%. It was applied on the arm, and the arm was dry after 2-3 minutes. No obvious yellowing phenomenon, easy to wash off with water. In the microbial sterilization test, the average kill log value of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com