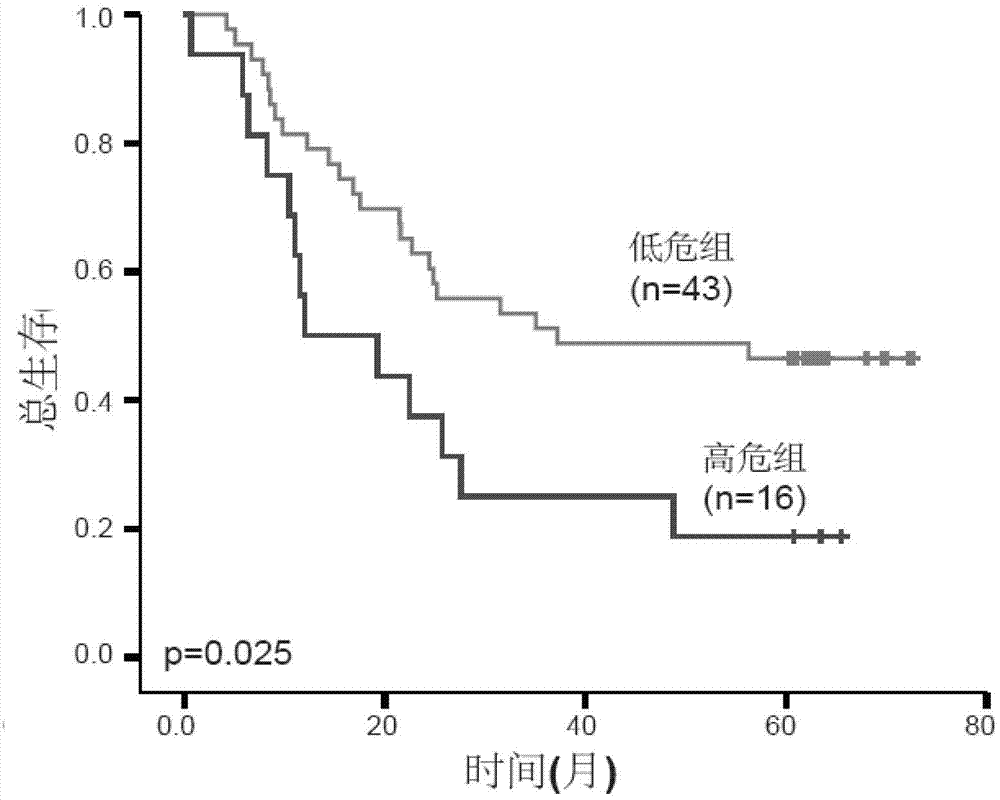
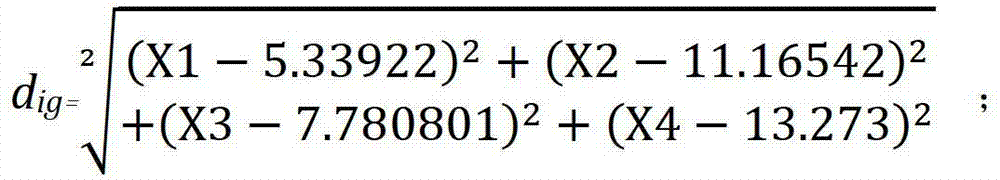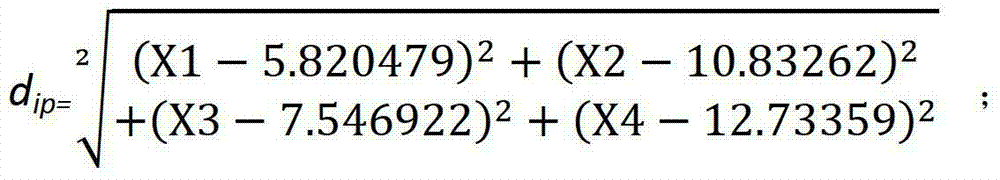Product for performing assisted prediction on postoperative survival time length of esophageal squamous carcinoma patient
A technology for esophageal squamous cell carcinoma and survival time, which is used in the field of products to assist in predicting the postoperative survival time of patients with esophageal squamous cell carcinoma.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1, establishment of the inventive method
[0031] 1. Obtaining specimens and survival information
[0032] 119 patients with primary esophageal squamous cell carcinoma who had undergone esophageal squamous cell carcinoma resection in the Cancer Institute of the Chinese Academy of Medical Sciences and adjacent normal tissues (frozen in liquid nitrogen and then transferred to a -80°C refrigerator) were used as specimens; all patients None of them received radiotherapy or chemotherapy before operation; the survival information of the patients was obtained through telephone follow-up. Informed consent was obtained from each patient.
[0033] For each specimen, a portion was reserved for paraffin embedding, sectioned, and routinely stained with H&E for pathological examination. The pathological features of each specimen were independently tested by two case physicians and were consistent, and the proportion of cancer cells in all tumor specimens was above 60%. ...
Embodiment 2
[0073] Embodiment 2, the application of the inventive method
[0074] The cancer tissues of another 47 esophageal squamous cell carcinoma patients (patients to be tested) who underwent esophageal squamous cell carcinoma resection in the Cancer Institute of the Chinese Academy of Medical Sciences were collected, and the postoperative survival time of each patient to be tested was predicted according to the following steps:
[0075] 1. Total RNA extraction and reverse transcription
[0076] According to the method in step 2 of Example 1, the total RNA of the cancer tissues of each patient to be tested was extracted respectively.
[0077] 2. Detection of miRNA expression
[0078] The expression levels of 178 miRNAs shown in Table 2 in the cancer tissue of the patient to be tested were detected according to the method in step 3 of Example 1.
[0079] 3. Data normalization processing
[0080] Rank the above 178 miRNA expression data of each patient's cancer tissue obtained in st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com