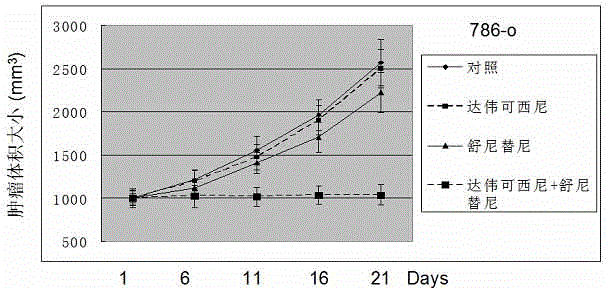
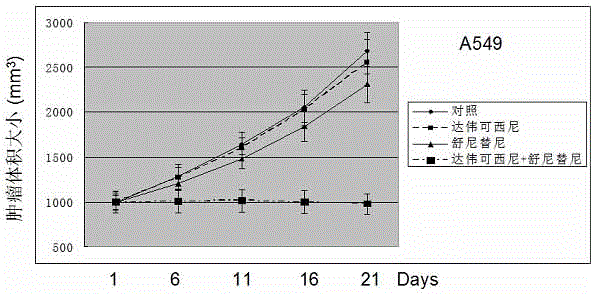
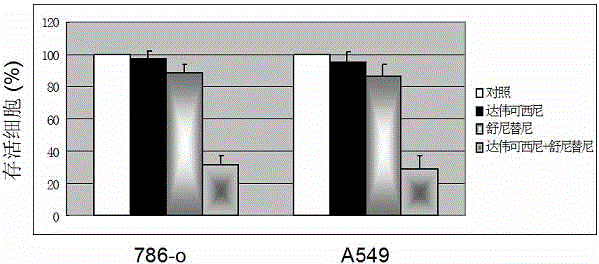
Anticancer composition based on anti-angiogenesis medicine
An anti-vascular and composition technology, applied in the field of tumor treatment, can solve the problems such as incomplete effect, not durable, drug resistance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1 Preparation of antitumor pharmaceutical composition
[0017] The formula is as follows:
[0018] Sunitinib 40 mg
[0019] Davacosini 40mg
[0020] 1N HCl 940 µl
[0021] Tween 80 50 microliters
[0022] 5 mL of 50 mM citrate buffer
[0023] 1% polyethylene glycol PEG-300 1ml
[0024] Sterile water Add to total volume to 10ml
[0025] The preparation method is:
[0026] Add 40 mg of sunitinib to 940 μl of 0.1N HCl, add 50 μl of Tween 80, add 5 ml of 50 mM citrate buffer, and 1 ml of 1% polyethylene glycol PEG-300, Finally, add water to 10 ml and shake vigorously to dissolve it; add 40 mg of Davicocinib to the above 40 mg of sunitinib / 10 ml.
Embodiment 2
[0028] The formula is as follows:
[0029] Sunitinib 10mg
[0030] Davacosini 40mg
[0031] 1N HCl 940 µl
[0032] Tween 80 50 microliters
[0033] 5 mL of 50 mM citrate buffer
[0034] 1% polyethylene glycol PEG-300 1ml
[0035] Sterile water Add to total volume to 10ml
[0036] The preparation method is:
[0037] Add 10 mg of sunitinib to 940 μl of 0.1N HCl, add 50 μl of Tween 80, add 5 ml of 50 mM citrate buffer, and 1 ml of 1% polyethylene glycol PEG-300, Finally, add water to 10 ml and shake vigorously to dissolve it; add 40 mg of Davicocinib to the above 10 mg of sunitinib / 10 ml.
Embodiment 3
[0039] The formula is as follows:
[0040] Sunitinib 70 mg
[0041] Davacosini 40mg
[0042] 1N HCl 940 µl
[0043] Tween 80 50 microliters
[0044] 5 mL of 50 mM citrate buffer
[0045] 1% polyethylene glycol PEG-300 1ml
[0046] Sterile water Add to total volume to 10ml
[0047] The preparation method is:
[0048]Add 70 mg of sunitinib to 940 μl of 0.1N HCl, add 50 μl of Tween 80, add 5 ml of 50 mM citrate buffer, and 1 ml of 1% polyethylene glycol PEG-300, Finally, add water to 10 ml and shake vigorously to dissolve it; add 40 mg of Davicocinib to the above 70 mg of sunitinib / 10 ml.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com