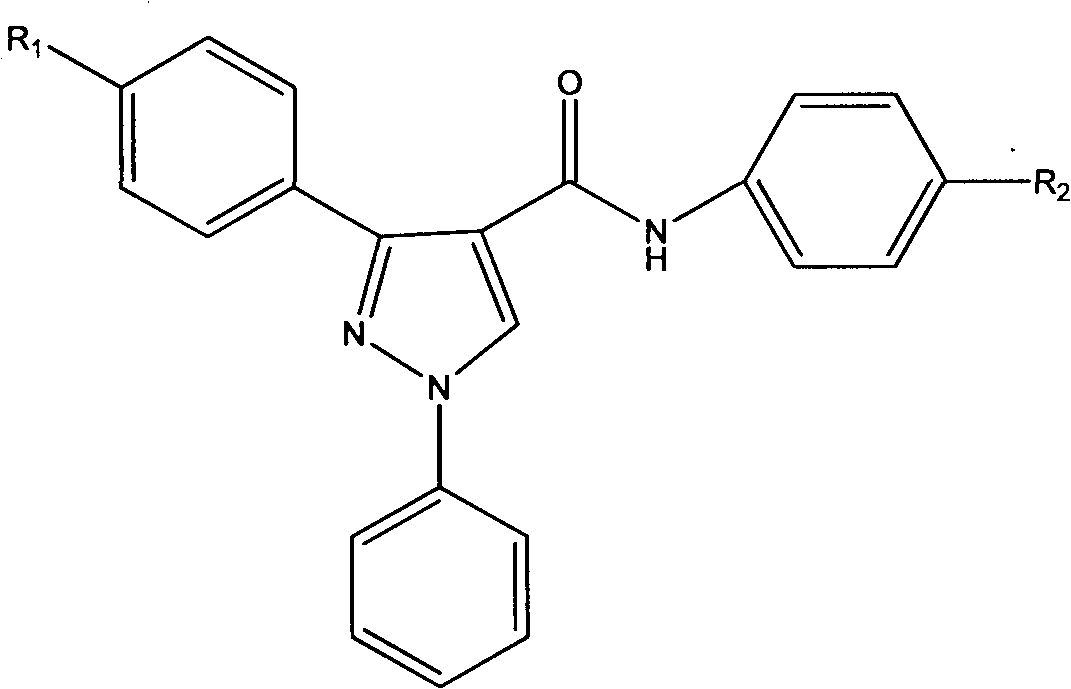
Preparation method of diarylpyrazolylaniline compounds, and application of compounds in colorectal cancer treatment
A technology of diarylpyrazoleanilines and compounds, applied in the preparation of diarylpyrazoleanilines and their application in the treatment of colon cancer, which can solve the problems of low clinical cure rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
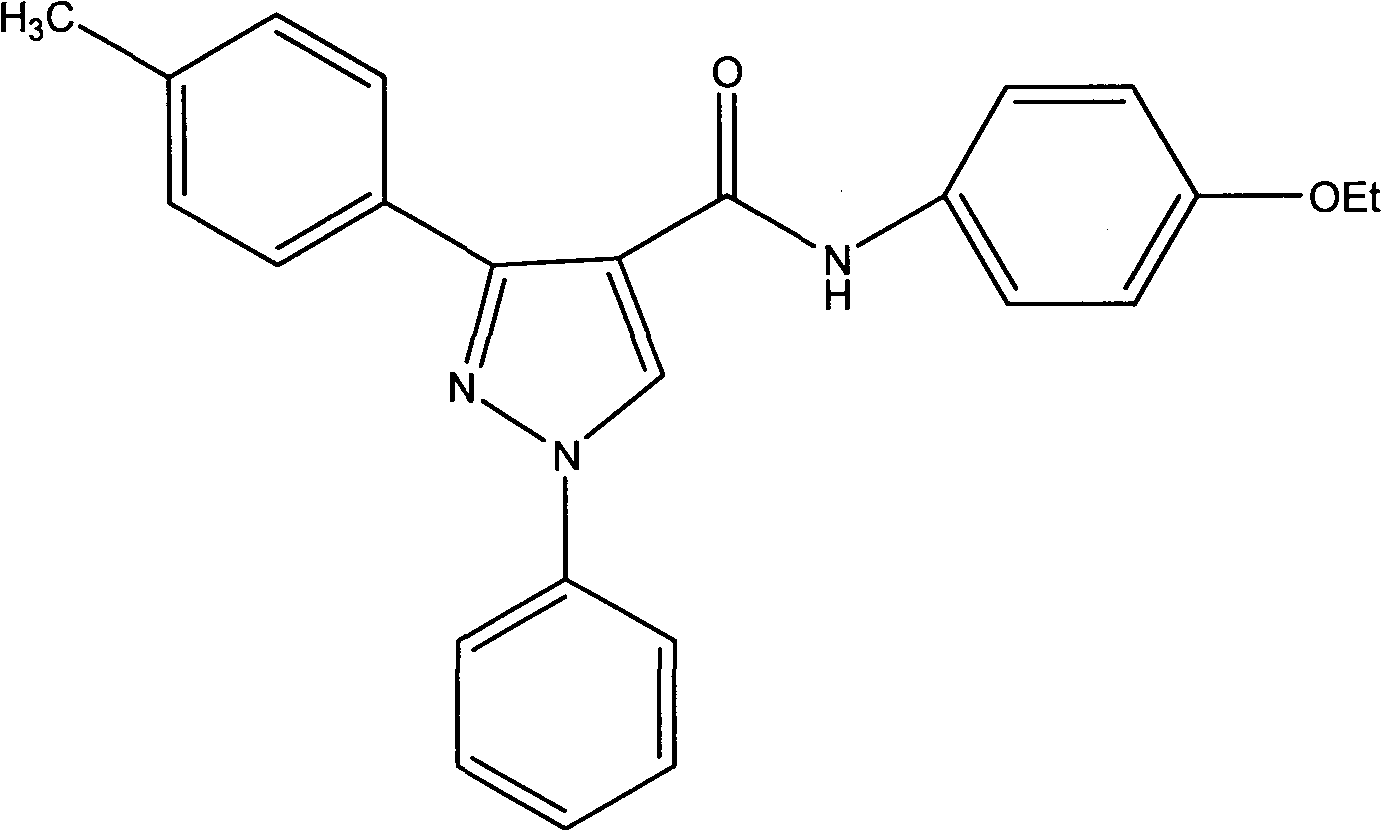
[0017] Example 1: Preparation of N-(4-oxyethylphenyl)-3-(4-methylbenzene)-1-phenyl-1H-pyrazole-4-aniline (compound 1)
[0018]
[0019] Dissolve p-methylacetophenone (20mmol), phenylhydrazine hydrochloride (25mmol) and anhydrous sodium acetate (40mmol) in ethanol respectively, stir magnetically, and react at 50-60°C for 4h (TLC to check the progress of the reaction). After the completion of the reaction, the reaction solution was spin-dried, ethyl acetate and water were extracted 2-3 times, and the organic layer was spin-dried to obtain a solid. Combine DMF and POCl 3 (12ml) Mix in an ice bath for 15 minutes, then dissolve the above solid in 3ml DMF and add dropwise to the above mixed solution, and gradually transfer to room temperature to 70-80°C for 5 hours. After cooling to normal temperature, pour into ice water, adjust pH to 7-8 with 50% NaOH, filter with suction and wash with water 3 times, and dry to obtain a solid. The resulting product was dissolved in acetone (30ml), a...
Embodiment 2
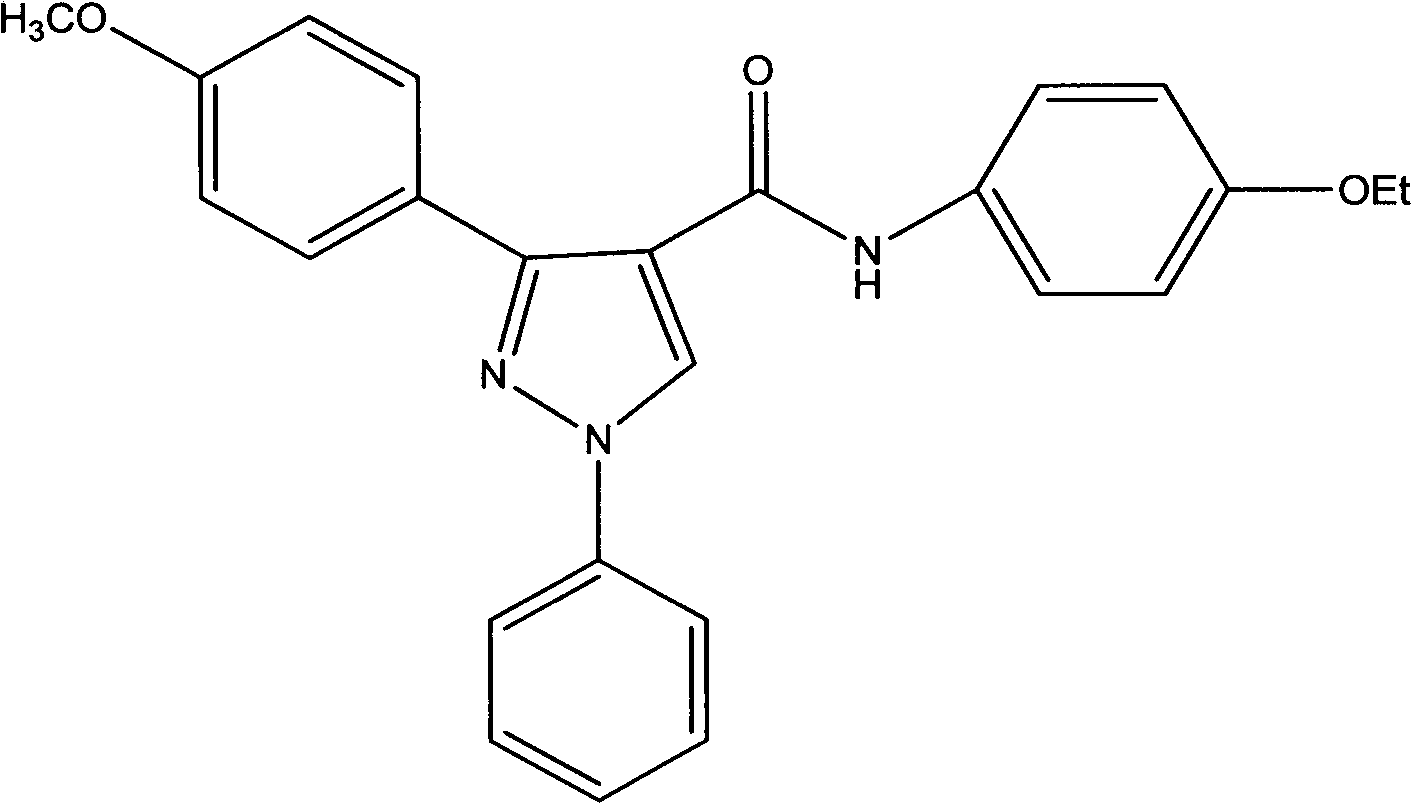
[0020] Example 2: Preparation of N-(4-oxyethylphenyl)-3-(4-oxymethylbenzene)-1-phenyl-1H-pyrazole-4-aniline (compound 2)
[0021]
[0022] The preparation method is the same as in Example 1. Substituting p-oxymethylacetophenone for the p-methylacetophenone in Example 1 to obtain the target compound in white crystal form. The yield is 68%, mp: 232-234°C; 1H NMR (300MHz, DMSO-d6, δppm): 8.54 (s, 1H); 7.60 (d, J = 8.12 Hz, 2H); 7.54 (d, J = 9.23 Hz, 4H); 7.50 (m, 3H); 7.35 (m, 3H); 6.84 (d, J = 8.79 Hz, 2H); 4.01 (dd, J = 14.07 Hz, 2H); 3.88 (s, 3H); 1.39 (t, J = 6.97 Hz, 3H). MS (ESI): 413.17 (C 25 H 24 N 3 O 3 [M+H]+).Anal.Calcd for C 25 H 23 N 3 O 3 : C, 72.62; H, 5.61; N, 10.16; O, 11.61. Found: C, 75.42; H, 5.56; N, 10.43; O, 11.46
Embodiment 3
[0023] Example 3: Preparation of N-(4-chlorophenyl)-3-(4-chlorobenzene)-1-phenyl-1H-pyrazole-4-aniline (compound 3)
[0024]
[0025] The preparation method is the same as in Example 1. Substitute p-chloroacetophenone for p-methylacetophenone in Example 1, and p-chloroaniline for p-ethoxyaniline in Example 1, to obtain the target compound in white crystal form. Yield 76%, melting point: 187-188°C; 1H NMR (300MHz, DMSO-d6, δppm): 8.56 (s, 1H); 7.72 (m, 4H); 7.52 (m, 4H); 7.29 (m, 7H) ).MS(ESI):408.28(C 22 H 16 Cl 2 N 3 O[M+H]+).Anal.Calcdfor C 22 H 15 Cl 2 N 3 O; C, 64.72; H, 3.70; Cl, 17.37; N, 10.29; O, 3.92. Found: C, 64.54; H, 3.66; Cl, 17.23; N, 10.15; O, 3.84.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com