Drug eluting balloon for the treatment of stenosis and method for manufacturing the balloon
一种球囊、药物的技术,应用在医药配方、药物输送、胶囊输送等方向,能够解决损失药物等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
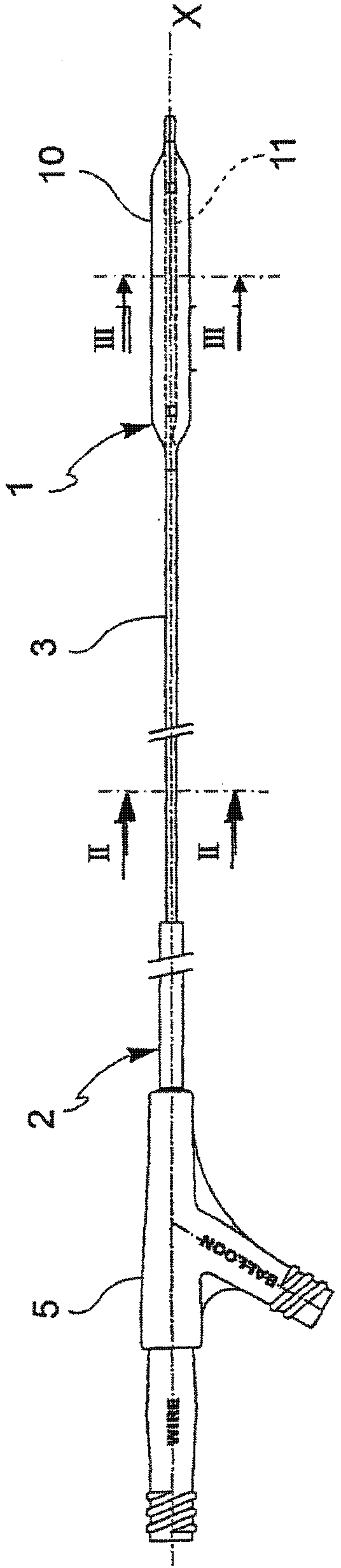
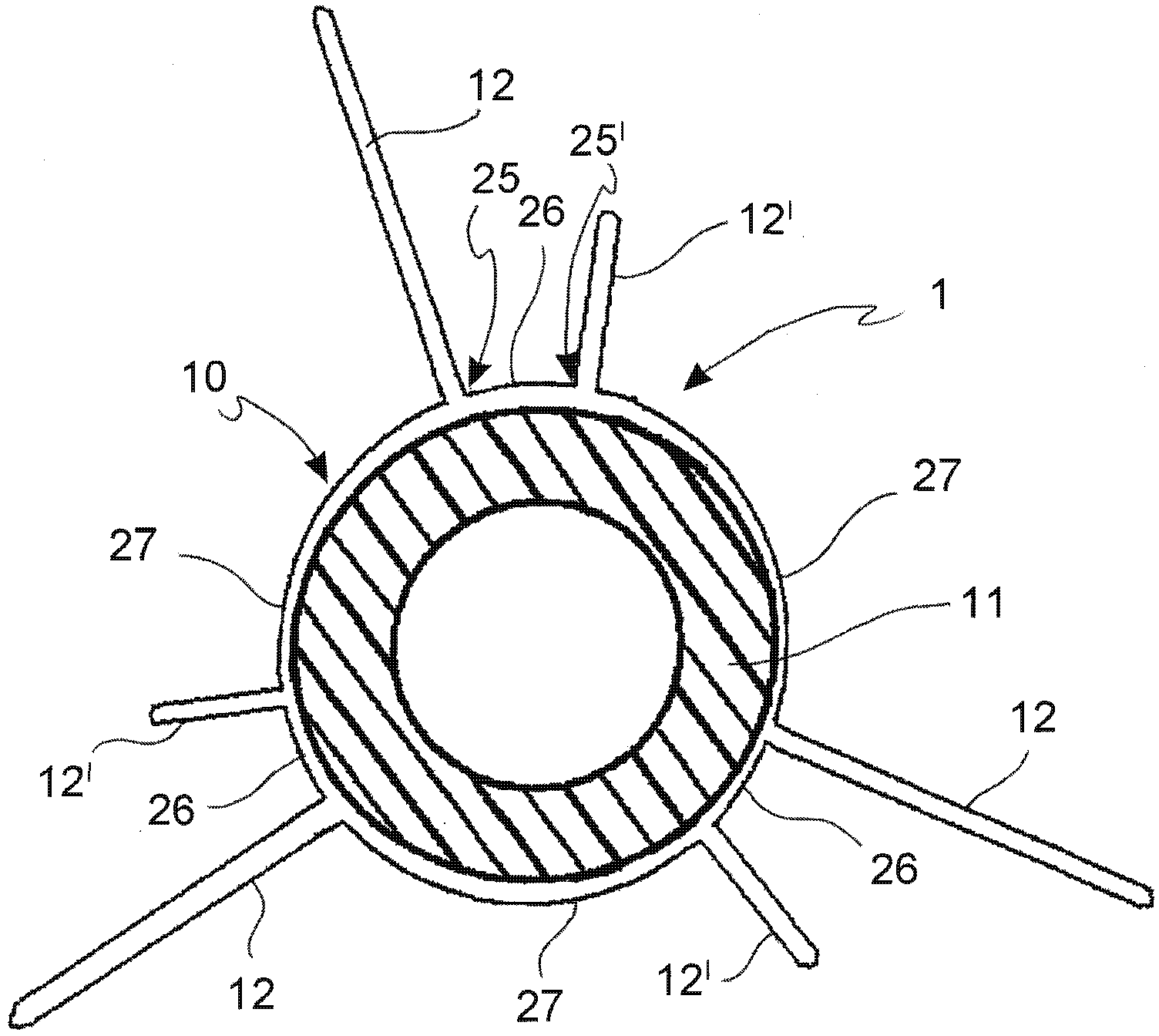
[0063] Referring to the drawings, an angioplasty balloon indicated by numeral 1 is mounted at the distal end of catheter 2 .
[0064] The catheter 2 also comprises an elongated tubular body 3 provided with a plurality of lumens 4, 4' (for a guide wire and a channel for inflating or deflating the balloon, respectively), and at its proximal end a connector means 5 . In the figures, the lumens 4, 4' are positioned side by side, but a coaxial arrangement is also conceivable.
[0065] The balloon 1 is adapted to adopt alternatively a deployed configuration and a deflated configuration. The balloon assumes the expanded configuration by injection of pressurized inflation fluid, and conversely, the balloon assumes the deflated configuration by suction of the same inflation fluid.
[0066] The angioplasty balloons of the present invention may be fabricated from any polymeric material conventionally used for these applications, such as, but not limited to, polyamide materials, blends ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com