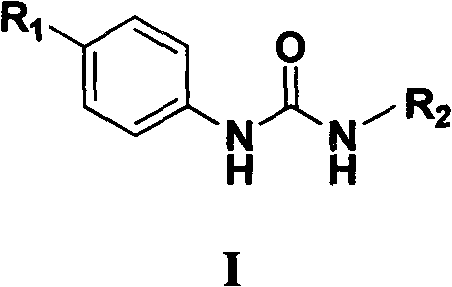
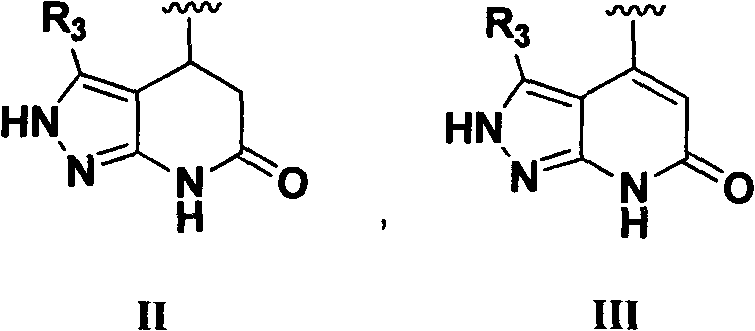
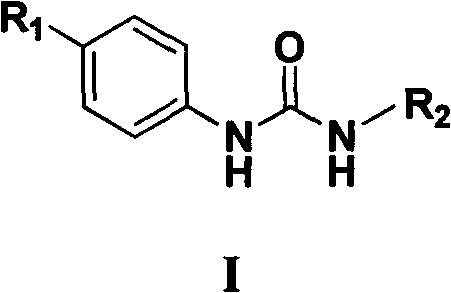
N,N'-aryl substituted urea compound and application thereof
A compound, a technology of substituted urea, applied in the field of medicinal chemistry and pharmacotherapeutics, can solve the problems of inability to obtain the effect of anti-tumor angiogenesis, long treatment period, and high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 3-Methyl-4-(4-nitrophenyl)-4,5-dihydro-2H-pyrazol[3,4-b]pyridin-6(7H)-one (Intermediate IV-1) to prepare
[0039]
[0040] 0.97 g of 3-methyl-5-amino-1H-pyrazole, 1.51 g of p-nitrobenzaldehyde and 1.44 g of 2,2-dimethyl-1,3-dioxane-4,6-dione Put it into a 50 ml round bottom flask, dissolve it with 5 ml of N,N-dimethylformamide, heat to reflux until there is no CO 2 until released. A solid precipitated out of the solution, and after cooling to room temperature, 20 ml of isopropanol was added for dilution. Suction filtration, the solid was washed with a mixed solution of N,N-dimethylformamide and isopropanol (N,N-dimethylformamide:isopropanol=1:2), and dried to obtain the title compound, 1.72 g White solid, yield 63%.
[0041] 1 H-NMR (400MHz, DMSO-d 6 )δ: 1.86(s, 3H), 2.58(dd, J 1 =16Hz,J 2 =5.6Hz), 2.88(dd, J 1 =16Hz,J 2 =7.2Hz, 1H), 4.36(t, J=6.4Hz, 1H), 7.51(d, J=8.8Hz, 2H), 8.19(d, J=8.8Hz, 2H), 10.39(s, 1H), 11.90 (s, 1H).
Embodiment 2
[0043] Preparation of 4-(4-nitrophenyl)-4,5-dihydro-2H-pyrazol[3,4-b]pyridin-6(7H)-one (intermediate IV-2)
[0044]
[0045] Put 0.83 g of 5-amino-1H-pyrazole, 1.51 g of p-nitrobenzaldehyde and 1.44 g of 2,2-dimethyl-1,3-dioxane-4,6-dione into a 50 ml round bottom flask , after dissolving with 5 ml of N,N-dimethylformamide, heat the reaction under reflux until there is no CO 2 until released. A solid precipitated out of the solution, and after cooling to room temperature, 20 ml of isopropanol was added for dilution. Suction filtration, the solid was washed with a mixed solution of N,N-dimethylformamide and isopropanol (N,N-dimethylformamide:isopropanol=1:2), and dried to obtain the title compound, 1.53 g White solid, yield 59%.
[0046] 1 H-NMR (400MHz, DMSO-d 6 )δ: 2.63 (dd, J 1 =15.6Hz,J 2 =7.2Hz, 1H), 2.83(dd, J 1 =16Hz,J 2 =6.8Hz, 1H), 4.43(t, J=7.2Hz, 1H), 7.34(s, 1H), 7.51(d, J=8.4Hz, 2H), 8.20(d, J=8.4Hz, 2H), 10.49(s, 1H), 12.20(s, 1H).
Embodiment 3
[0048] Preparation of 3-methyl-4-(4-aminophenyl)-4,5-dihydro-2H-pyrazol[3,4-b]pyridin-6(7H)-one (intermediate V-1)
[0049]
[0050] 1.36 g of intermediate IV-1 was dissolved in 20 ml of methanol, and 136 mg of 10% Pd / C was added for catalytic hydrogenation. React at room temperature for 5 hours. After the reaction, the reaction solution was adjusted to pH=4 with dilute hydrochloric acid, and then the Pd / C was removed by suction filtration. NaHCO 3 After the filtrate was adjusted to pH > 7, a solid precipitated out, filtered with suction, washed with water, and dried to obtain 0.91 g of a white solid (intermediate V-1), with a yield of 66%.
[0051] 1 H-NMR (400MHz, DMSO-d 6 )δ: 1.82(s, 3H), 2.48(dd, J 1 =16Hz,J 2 =6.4Hz, 1H), 2.68(dd, J 1 =16Hz,J 2 =6.8Hz, 1H), 3.93(t, J=6.4Hz, 1H), 4.93(s, 2H), 6.48(d, J=8.4Hz, 2H), 6.81(d, J=8.4Hz, 2H), 10.21(s, 1H), 11.73(s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com