Preparation method of Olmesartan Medoxomil
A technology of olmesartan medoxomil and compounds, which is applied in the field of preparation of olmesartan medoxomil, can solve the problems of low yield, achieve high conversion rate, accelerate reaction speed, and facilitate industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0056] In order to make the technical means, creative features, work flow, and use method of the present invention easy to understand and understand the purpose and effect of the present invention, the present invention will be further described with reference to specific embodiments.
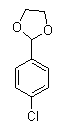
[0057] Synthesis of compound 4:
[0058] Under the protection of nitrogen, add 31.2g of magnesium powder (1.30mol) and 5.6g of iodine (0.022mol) into the reaction flask, heat until the iodine vapor is full of the reaction flask, and then add 18.5g of p-chlorophenylglycol formal (compound 3 ) (0.10mol) and 50ml of tetrahydrofuran, then stir to reflux, then add 2.54g of iodine (0.01mol), 184.5g of p-chlorophenyl glycol formal (compound 3) (1.00mol), 200ml
[0059] Tetrahydrofuran and 200ml of toluene were reacted at reflux for 5 hours. The reaction solution was cooled to room temperature and allowed to stand under the protection of nitrogen to obtain a grid (1.00mol), 13.9g anhydrous manganese chlori...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com