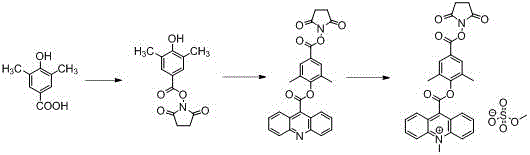
Synthesis of acridinium ester chemiluminescent substrate DMAE.NHS
A technology of chemiluminescence and chemiluminescent reagents, which is applied in the field of synthesis of acridine ester chemiluminescent substrates DMAE·NHS chemiluminescent reagents, can solve the problems of complex synthesis, many steps, and potential toxicity, and achieve simple reaction steps, The effect of mild reaction conditions and high overall yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0010] Example 1: Synthesis of 1-((4-hydroxyl-3,5-dimethylbenzoyl)oxy)-2,5-pyrrolidinedione
[0011] Dissolve 1.38 g (12 mmol) of N-hydroxysuccinimide and 2.44 g (11.07 mmol) of dicyclohexylcarbodiimide in anhydrous DMF, cool to about 0 °C, and dissolve 1.53 g (9.22 mmol) of 3, 5-Dimethylbenzoic acid was dissolved in 20mL DMF and placed in a constant pressure dropping funnel, slowly added dropwise, the temperature of the reaction system was controlled not to be higher than 5°C, after the dropwise addition was completed, the reaction was continued and the temperature was slowly raised to room temperature. After the reaction was complete, a certain amount of water was added, a large amount of white solids were precipitated, suction filtered, the filter cake was washed with ethyl acetate, the filtrate was extracted 3 times with ethyl acetate, washed once with water, dried, and spin-dried to obtain a crude product, all The solid was recrystallized from ethyl acetate to obtain 2.1 ...
specific Embodiment 2
[0012] Specific Example 2: Preparation of 2', 6'-dimethyl-4'-(N-succinimidyl) phenyl-acridine-9-carboxylate
[0013] 11 g (49.38 mmol) acridine-9-carbonyl chloride, 10 g (37.99 mmol) 1-((4-hydroxy-3,5-dimethylbenzoyl)oxy)-2,5-pyrrolidine Diketone and 7.24 g (37.99 mmol) p-toluenesulfonyl chloride were dissolved in 200 mL of anhydrous pyridine; under a nitrogen atmosphere, heated to 100 ° C. After the reaction is complete, the solvent is spin-dried, and after spin-drying, add 50mL of 1N hydrochloric acid (1N means that 1mL of concentrated hydrochloric acid is diluted with water to form a 12mL solution), 200mL of saturated sodium chloride solution, and 50mL of 1% sodium hydroxide solution. Ethyl acetate was extracted three times, the organic phase was washed three times with water, dried and spin-dried to obtain 7.2 g of crude product, and the obtained solid was purified by column chromatography to obtain 5 g of product. The yield was 28.1%.
Embodiment 3
[0014] Embodiment 3: the preparation of acridinium ester DMAE NHS
[0015] Add 1 g (2.13 mmol) of 2',6'-dimethyl-4'-(N-succinimidyl)phenyl-acridine-9-carboxylate into 20 mL of anhydrous toluene, heat to 110°C It was completely dissolved in toluene and cooled to room temperature. After cooling slightly, 11 mL of dry redistilled dimethyl sulfate was added. Under the protection of nitrogen atmosphere, the reaction was stirred at 110°C for 20 hours, and the solution was cooled to room temperature, followed by 4 Continue to stir the reaction at ℃ for 1 hour; filter the obtained mixture, wash the solid twice with toluene, then dissolve the yellow solid in 60 mL of dichloromethane, heat to boiling, keep for 2-3 minutes, and filter hot, the obtained filtrate Concentrate to about 40 mL, cool to room temperature to obtain yellow crystals, wash with ether three times, and obtain 500 mg of yellow solids after vacuum drying with a yield of 39.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com