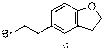
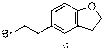
Synthetic technology of darifenacin intermediate 5-(2-bromomethyl)-2,3-dihydro-1-coumarone
A technology of alkyl and carbonyl, applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of unsuitable industrialization, high cost, low yield, etc., achieve high industrial application and economic value, increase the total yield, and improve the yield high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
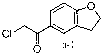
[0036] Embodiment 1: the preparation of formula a-1 compound
[0037]
[0038] Put anhydrous aluminum trichloride (215g, 1.62mol) and 770ml of dichloromethane into the reaction bottle under the condition of -5~5℃, add acetyl chloride (95g, 1.22mol) dropwise, after the dropwise add 2, 3-Dihydrobenzofuran (97 g, 0.81 mol), continued the reaction for 2 to 2.5 hours after the dropwise addition was completed. After the reaction was completed, 1.5 L of ice water was added, the organic layer was separated, and washed successively with 400 ml of dilute hydrochloric acid and 400 ml of water. The organic layer was separated, dried over anhydrous magnesium sulfate, and concentrated. 200ml of n-hexane was added for recrystallization, and a white solid was precipitated, which was dried to obtain 120g of the compound of formula a-1 as a white solid, with a yield of 75.9%.
[0039]
Embodiment 2
[0040] Embodiment 2: the preparation of formula b-1 compound
[0041]
[0042] Under nitrogen protection, add compound a-1 (5g, 0.025mol), sodium acetate (4.1 g, 0.05mol) and 50ml N,N-dimethylformamide, sodium iodide (0.1g, 0.6mmol), heat up to 120 °C, followed by TLC until the reaction was complete. 50 ml of ethyl acetate was added for extraction, the organic phase was washed twice with 50 ml of water, and the organic layer was concentrated to obtain 5.1 g of the compound of formula b with a yield of 92.7%.
[0043] 1 HNMR (400MHz, CDC1 3 ) δ=2.22(s,3H), δ=3.23 (t, J = 7.2 Hz, 2H), δ=4.64 (t, J = 7.2 Hz,2H), δ=5.2(s, 2H), δ=6.81 (d, J=6.8Hz,2H), δ=7.72 (d, J=6.8Hz, 1H),7.80 (s, lH) ;
[0044] MS: 243 (M + Na).
Embodiment 3
[0045] Embodiment 3: the preparation of formula b-1 compound
[0046]Under the protection of nitrogen, add compound a-1 (5g, 0.025mol), acetic acid (3g, 0.05) and 50ml tetrahydrofuran, sodium iodide (0.1g, 0.6mmol) triethylamine (5.05g, 0.05), heat up to reflux, TLC followed until the reaction was complete. Concentrate to remove tetrahydrofuran, add 50 ml of ethyl acetate for extraction, wash with 50 ml of water twice, and concentrate the organic layer to obtain 4.9 g of the compound of formula b with a yield of 89.1%.
[0047]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com