Compound and medicament for treating MRSA (Methicillin-resistant Staphylococcus Aureus) infection
A compound and combined technology, applied in the field of compounds and drugs for the treatment of MRSA infection, can solve the problem of low specificity and achieve the effect of high specificity and low side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
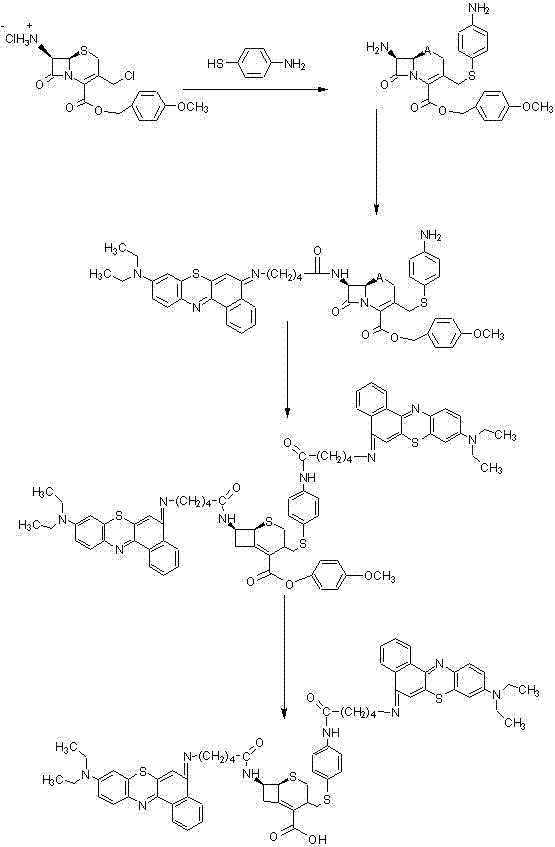
[0064] In this example, the synthetic raw material is: 7-amino-3-chloromethyl-3-cefoxime-4-carboxylic acid p-methoxyphenyl methyl ester (7-amino-3-chloromethyl-3-cephen-4 -carboxylic acid p-methoxybenzyl ester, hereinafter referred to as ACLE), photosensitizer 5-(4′-carboxybutylamino)-9-diethylaminobenzothiaphenazine chloride (5-(4′-carboxybutylamino)-9-diethylaminobezo [a] phenothiazinium chloride, hereinafter referred to as EtNBS-COOH). The above-mentioned raw materials have been commercialized, such as Santa Cruz Company, Sigma Company, Merck Company, etc. can be purchased, or can also be prepared according to the existing technology.
[0065] refer to figure 1 , the steps of synthesizing the compound in this example are as follows:
[0066] step 1
[0067] Add 30 mg of ACLE chloride to the dichloromethane solvent, add 15 μl of tripropylamine, 12 μl of N-methylimidazole, and 15 mg of 4-aminothiophenol, and react in an ice bath to obtain the compound 1- 1.
[0068] step...
Embodiment 2
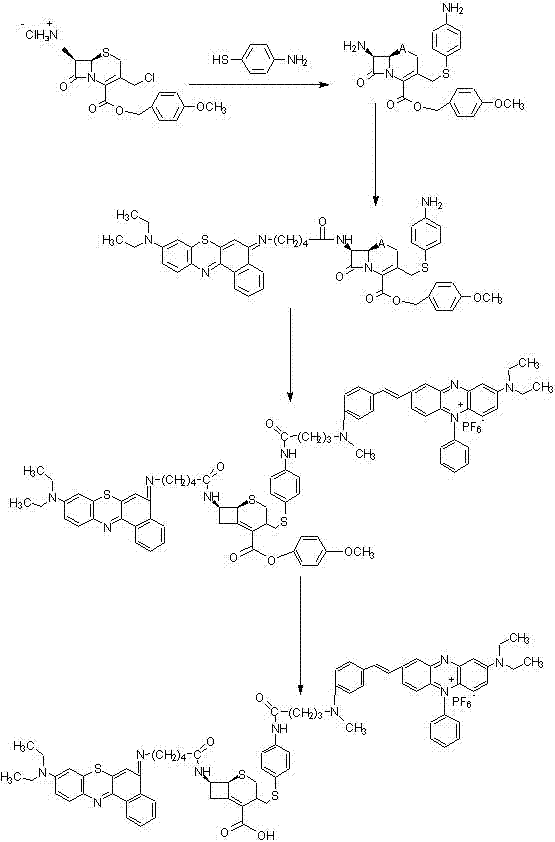
[0074] Under strict storage and use conditions, the introduced photosensitive group will not actively decompose, but in order to prevent the photosensitive group from decomposing without specific illumination, on the basis of Example 1, this example is in the target product A quencher is introduced to ensure product stability.
[0075] In this example, the synthetic raw material is: 7-amino-3-chloromethyl-3-cefoxime-4-carboxylic acid p-methoxyphenyl methyl ester (7-amino-3-chloromethyl-3-cephen-4 -carboxylic acid p-methoxybenzyl ester, hereinafter referred to as ACLE), photosensitizer 5-(4′-carboxybutylamino)-9-diethylaminobenzothiaphenazine chloride (5-(4′-carboxybutylamino)-9-diethylaminobezo [a] phenothiazinium chloride, hereinafter referred to as EtNBS-COOH), black hole quencher (Black Hole Quencher3, hereinafter referred to as BHQ3).
[0076] refer to figure 1 , the steps of synthesizing the compound in this example are as follows:
[0077] step 1
[0078] Add 30 mg o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com