SP35 antibodies and uses thereof
An antibody and monoclonal antibody technology, applied in the field of neurology, can solve unmet problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
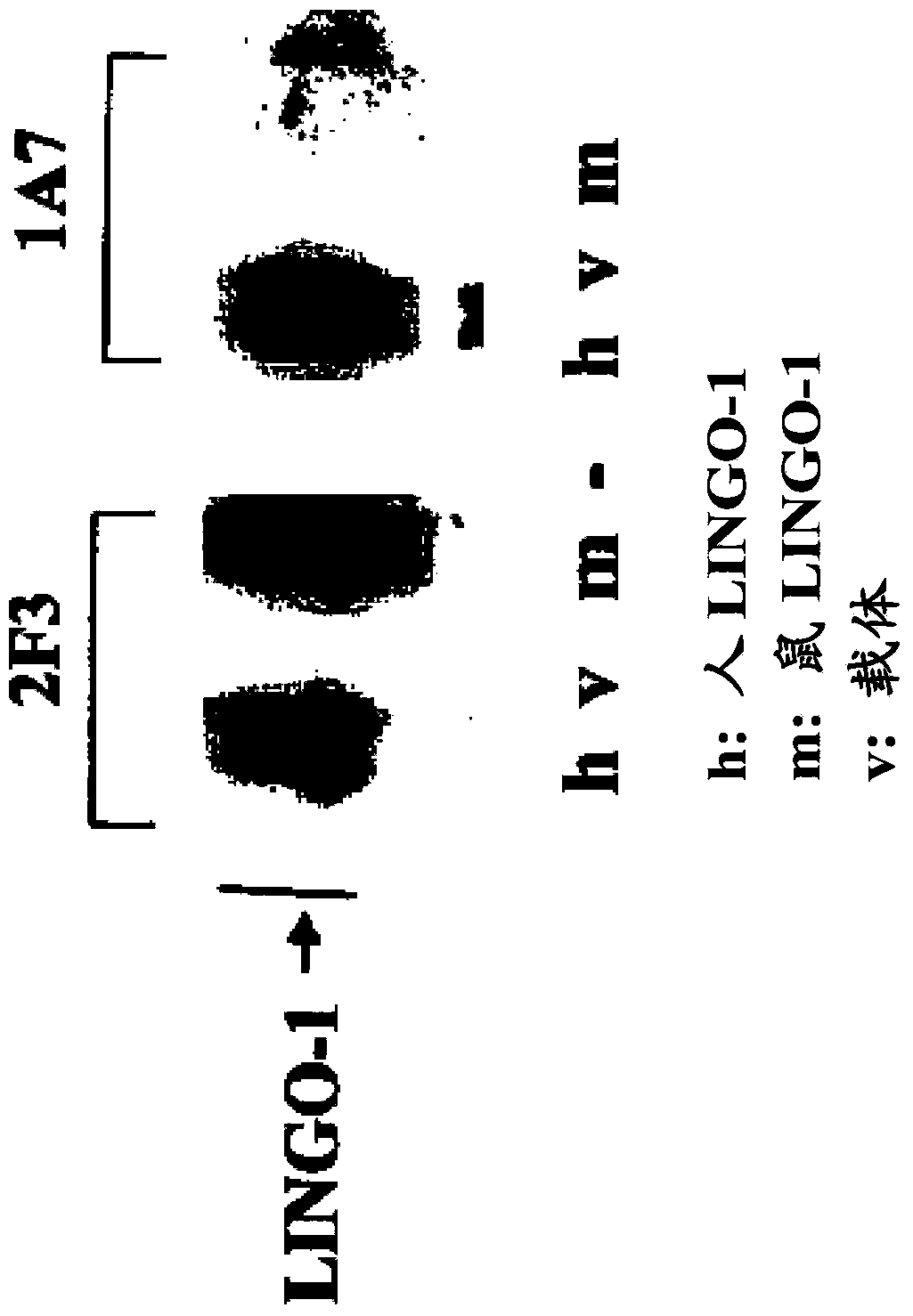
[0569] Sp35 is involved in oligodendrocyte biology
[0570] Oligodendrocyte maturation goes through multiple developmental stages, from A2B5 progenitors (expressing A2B5), to premyelinating oligodendrocytes (expressing O1 and O4) and finally to mature myelinating oligodendrocytes (expressing O1 , O4 and MBP). Thus, by monitoring the presence and absence of A2B5, O1, O4 and MBP markers, it is possible to determine the developmental stage of a given cell and assess the role of Sp35-Fc in oligodendrocyte biology. A general review of oligodendrocyte biology can be found, for example, in Baumann and Pham-Dinh, Physiol. Rev. 81:871-927 (2001).
[0571] Monoclonal antibodies against O4, MBP and CNPase were purchased from Sternberger Monoclonals; antibodies against APC (clone CC-1; ref.29) were purchased from Calbiochem. Other antibodies were directed against βIII-tubulin (Covance), Sp35 (Biogen Idee), Fyn (Santa Cruz Biotechnology) and phospho-Fyn (Biosource). Monoclonal antibod...
Embodiment 2
[0586] Construction and purification of Sp35-Fc protein
[0587] To study the biological function of Sp35, a construct can be prepared that fuses the extracellular portion of human Sp35 (residues 1-532) with the hinge and Fc region of human IgG1. The partial coding sequence of human Sp35 can be obtained by using forward primer 5'-CAG CAG GTC GAC GCG GCC GCA TGC TGG CGG GGG GCG T-3' (SEQ ID NO:354) and reverse primer 5'-CAG CAG GTC GAC CTC PCR for GCC CGG CTG GTT GGC CAA CCA GCC GGG CGA GGT CGA CCT CGA GG-3' (SEQ ID NO: 355) was obtained from clone 227.2.
[0588] This blunt ended PCR product was subcloned into the SrfI site of the PCR SCRIPT AMP vector (Stratagene) to create PCR SCRIPT AMP-Sp35. The SalI fragment was isolated from PCR SCRIPT AMP-Sp35 and subcloned into PCRCAMP Ig vector (from Stratagene vector PCR SCRIPT AMP). In the PCRCAMP Ig vector, the hinge and Fc gamma sequences were subcloned as SalI (5') into a NotI (3') fragment. This SalI Sp35 fragment was subclon...
Embodiment 3
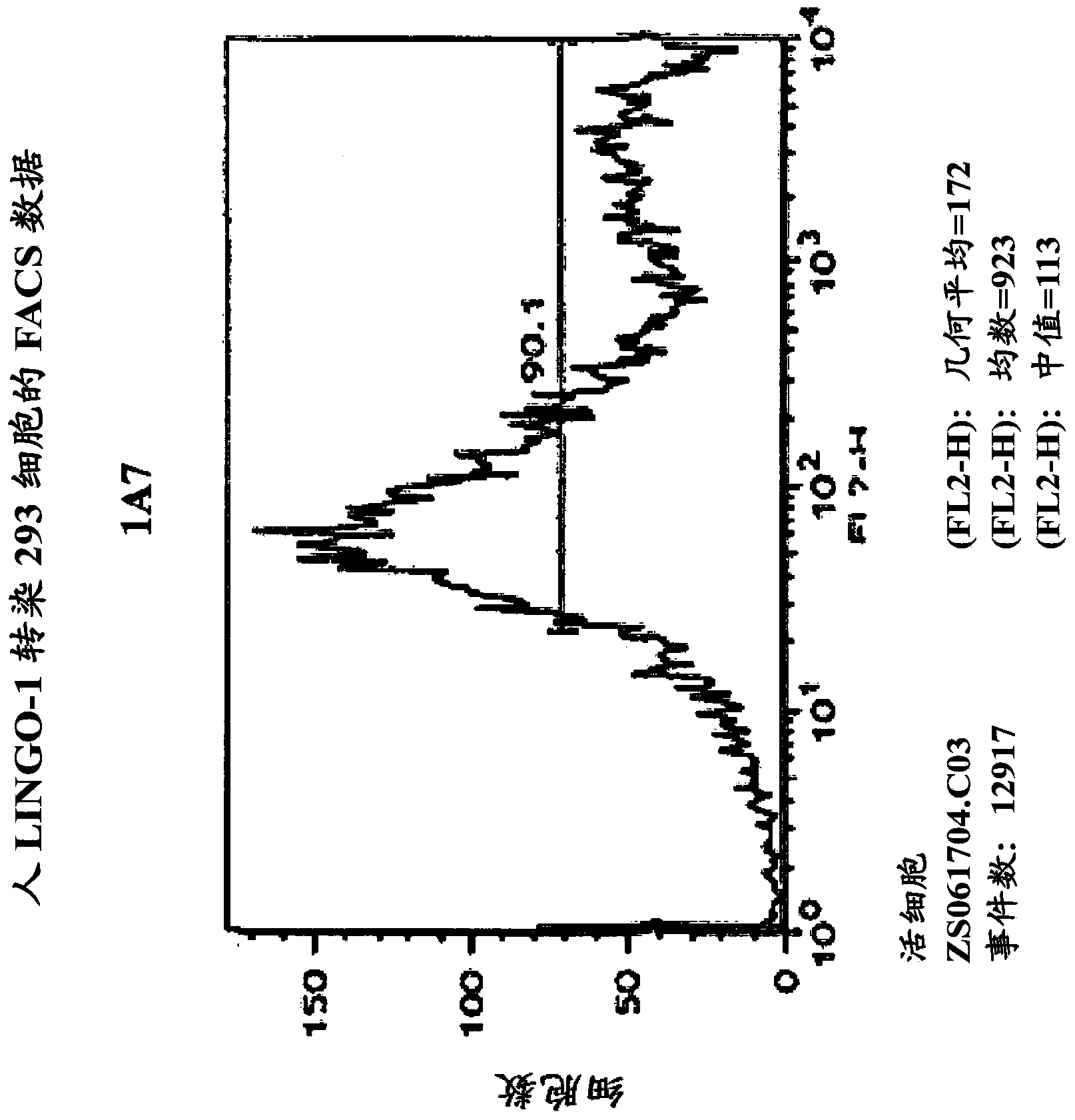
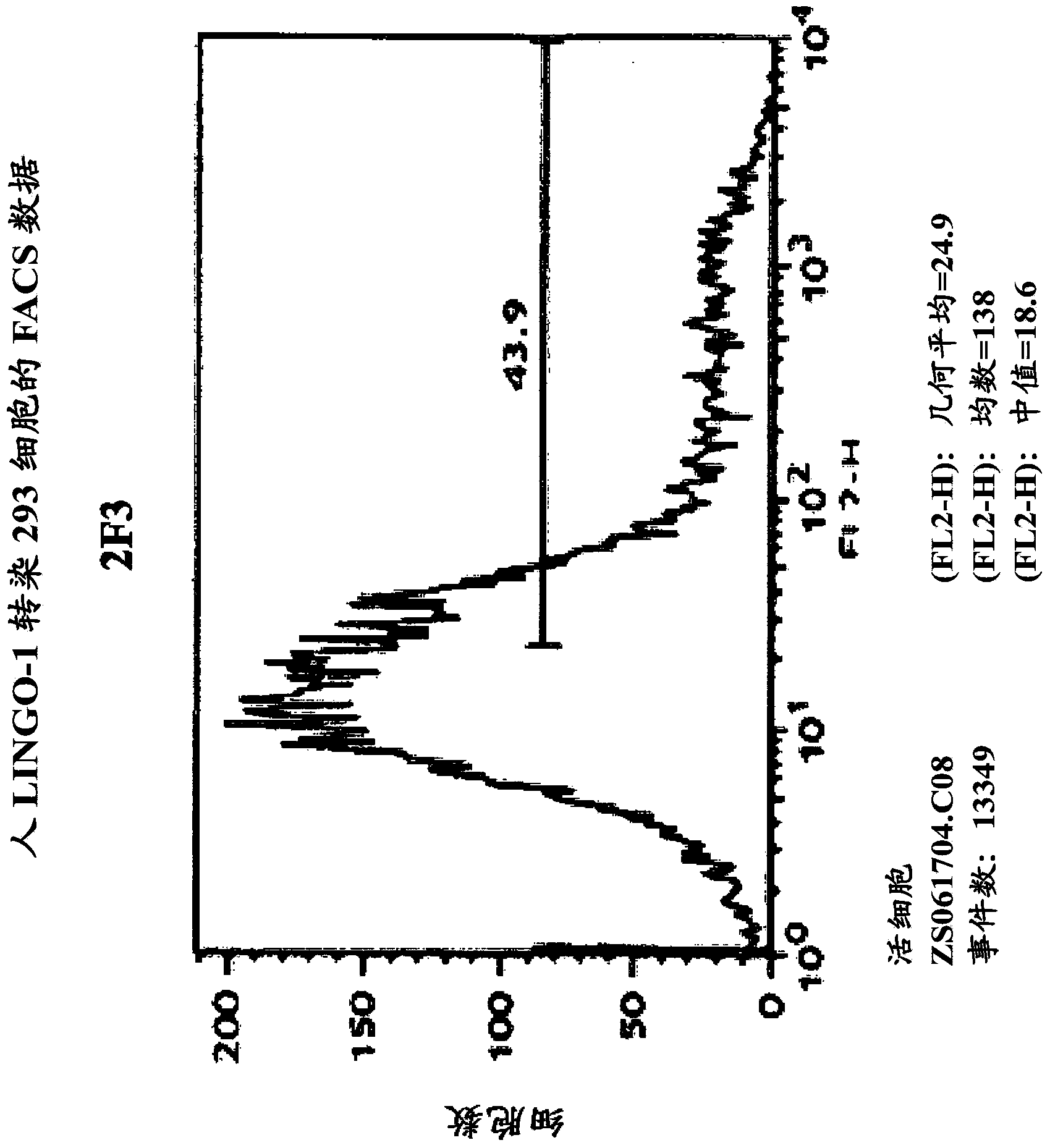
[0592] Production of Sp35-specific monoclonal antibodies
[0593] The anti-Sp35 antibody that specifically binds to the Sp35 polypeptide of the present invention can be prepared using the following methods and steps.
[0594] A. Antibody Screening Analysis
[0595] 1. ELISA analysis
[0596] 96-well MaxiSorp TM Tablet (Nunc TM ) was added to each microwell of Sp35-Fc (0.5 μg dissolved in 50 μl of 0.1 M sodium bicarbonate buffer at pH 9.0). Plates were incubated at 37°C for 1 hour or at 4°C for 16 hours. Non-specific binding sites on plates were blocked with 25 mM HEPES pH 7.4 containing 0.1% BSA, 0.1% ovalbumin, 0.1% (5% (w / v) nonfat dry milk in 150 mM NACE) and 0.001% azide point. Serum or dilutions of the hybridoma suspension (eg, serial three-fold dilutions) are added to each row of that on the plate, followed by incubation at 25°C for 1 hour. After washing three times with PBS, 50 μl of a 1:10,000 dilution of a horseradish peroxidase-conjugated goat anti-mouse secon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com